推荐产品
等級
pharmaceutical primary standard
API 家族
mepivacaine
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
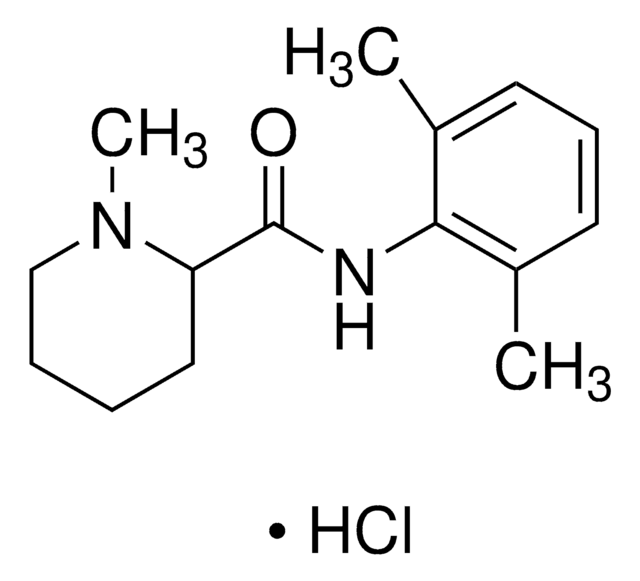
SMILES 字串
O=C(NC1=C(C)C=CC=C1C)C2NCCCC2
InChI
1S/C14H20N2O/c1-10-6-5-7-11(2)13(10)16-14(17)12-8-3-4-9-15-12/h5-7,12,15H,3-4,8-9H2,1-2H3,(H,16,17)
InChI 密鑰
SILRCGDPZGQJOQ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Mepivacaine impurity B EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
B Bruguerolle et al.
Clinical and experimental pharmacology & physiology, 21(12), 997-999 (1994-12-01)
1. This study was designed to document the acute toxicity of two metabolites of bupivacaine, desbutylbupivacaine (2,6, desbutylbupivacaine; PPX) and pipecolic acid in mice. All the compounds were administered by the intraperitoneal (i.p.) route. 2. The mean convulsant activity was
Mika J Jokinen et al.
European journal of clinical pharmacology, 58(10), 653-657 (2003-03-01)
To assess the effect of ciprofloxacin on the pharmacokinetics of ropivacaine. METHODS. In a double-blind, randomised, cross-over study, nine healthy volunteers were treated for 2.5 days with 500 mg oral ciprofloxacin or placebo twice daily. On day 3, they received
M Gantenbein et al.
The Journal of pharmacy and pharmacology, 49(3), 293-295 (1997-03-01)
Previous workers have reported that 0.01 mg kg-1 of levcromakalim injected intraperitoneally did not modify bupivacaine-induced neurotoxicity but increased the duration of action of bupivacaine. This study was designed to document possible changes in the pharmacokinetic behaviour of bupivacaine and
Robin Ledger
Journal of biochemical and biophysical methods, 57(2), 105-114 (2003-08-14)
Less than 11% of the dose of bupivacaine could be accounted for in urine from 10 patients receiving continuous epidural infusions. HPLC analysis of metabolites confirmed (S)-bupivacaine was more extensively metabolised than (R)-bupivacaine, and dealkylation was the predominant metabolic pathway
L Aarons et al.
British journal of anaesthesia, 107(3), 409-424 (2011-06-23)
The aim was to characterize ropivacaine and 2',6'-pipecoloxylidide (PPX) pharmacokinetics and factors affecting them in paediatric anaesthesia. Population pharmacokinetics of ropivacaine and its active metabolite PPX were estimated after single and continuous ropivacaine blocks in 192 patients aged 0-12 yr
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门