推荐产品
等級
pharmaceutical primary standard
API 家族
gliclazide
製造商/商標名
EDQM
mp
163-169 °C (lit.)
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
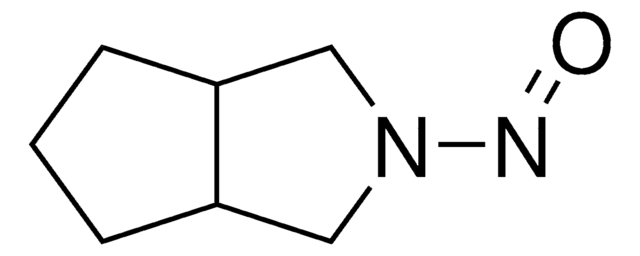
Cc1ccc(cc1)S(=O)(=O)NC(=O)NN2CC3CCCC3C2
InChI
1S/C15H21N3O3S/c1-11-5-7-14(8-6-11)22(20,21)17-15(19)16-18-9-12-3-2-4-13(12)10-18/h5-8,12-13H,2-4,9-10H2,1H3,(H2,16,17,19)
InChI 密鑰
BOVGTQGAOIONJV-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
用于治疗非胰岛素依赖型糖尿病(NIDDM)。
生化/生理作用
低密度脂蛋白(LDL)的氧化修饰在与糖尿病相关的血管功能障碍中起重要作用。格列齐特是具有清除自由基活性的第二代磺酰脲类药物。在增加浓度的格列齐特(1 至 10 μg/mL)存在下,将人主动脉平滑肌细胞(HASMC)与天然人 LDL(100 μg/mL)一起孵育会导致 HASMC 介导的 LDL 氧化剂量依赖性降低。HASMC暴露于格列齐特(1 至 10 μg/mL)和天然 LDL(100 μg/mL)中也导致氧化的 LDL 诱导的人类单核细胞与 HASMC粘附的剂量依赖性降低。此外,将 HASMC 与格列齐特一起孵育会大大降低氧化的 LDL 次级这些细胞增殖的能力。最后,在基因和蛋白质水平上,使用格列齐特处理 HASMC,将导致氧化修饰的 LDL 诱导的单核细胞趋化蛋白(MCP)-1 和人热休克蛋白 70(HSP 70)的表达显着降低。这些结果表明,格列齐特,浓度在治疗范围内(5 至 10 μg/mL)时,在体外可有效减轻氧化修饰的 LDL 引起的血管平滑肌细胞(VSMC)功能障碍。对 2 型糖尿病患者给予格列齐特可能成为预防和管理糖尿病心血管疾病策略的一部分
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
K J Palmer et al.
Drugs, 46(1), 92-125 (1993-07-01)
Gliclazide is a second generation sulphonylurea oral hypoglycaemic agent used in the treatment of non-insulin-dependent diabetes mellitus (NIDDM). It improves defective insulin secretion and may reverse insulin resistance observed in patients with NIDDM. These actions are reflected in a reduction
Jane K McGavin et al.
Drugs, 62(9), 1357-1364 (2002-06-22)
Gliclazide modified release (MR) is a new formulation of the drug gliclazide and is given once daily. The hydrophilic matrix of hypromellose-based polymer in the new formulation effects a progressive release of the drug which parallels the 24-hour glycaemic profile
Metabolic approach to atherosclerosis: effect of gliclazide.
D N Brindley
Metabolism: clinical and experimental, 41(5 Suppl 1), 20-24 (1992-05-01)
A Harrower
Metabolism: clinical and experimental, 49(10 Suppl 2), 7-11 (2000-11-15)
Although sulfonylureas have been used for more than 40 years, it is only recently that their molecular mechanisms of action have been elucidated. Gliclazide modified release, whose introduction comes soon after the sequencing and cloning of the sulfonylurea receptor, is
K G Alberti
Diabete & metabolisme, 20(3 Pt 2), 341-348 (1994-11-01)
Gliclazide is a second-generation sulfonylures that is widely used in the treatment of non-insulin-dependent diabetes mellitus (Type 2 diabetes). It has been recommended for use on the basis of both its metabolic and nonmetabolic effects. It has a clear beneficial
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门