所有图片(1)
About This Item
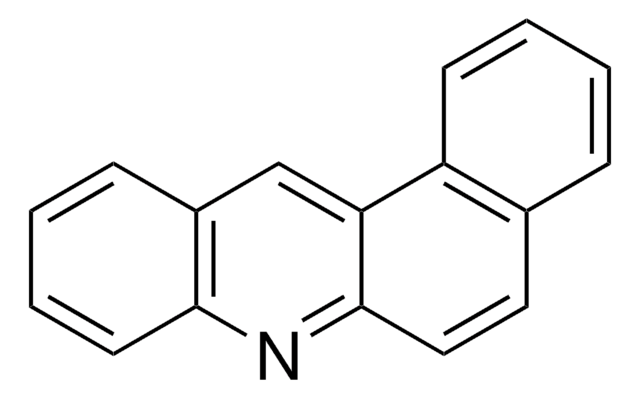
经验公式(希尔记法):
C17H11N
CAS号:
分子量:
229.28
Beilstein:
9262
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推荐产品
等級
certified reference material
agency
BCR®
製造商/商標名
JRC
技術
HPLC: suitable
gas chromatography (GC): suitable
格式
neat
儲存溫度
2-8°C
SMILES 字串
c1ccc2nc3ccc4ccccc4c3cc2c1
InChI
1S/C17H11N/c1-3-7-14-12(5-1)9-10-17-15(14)11-13-6-2-4-8-16(13)18-17/h1-11H
InChI 密鑰
JEGZRTMZYUDVBF-UHFFFAOYSA-N
分析報告
For more information please see:
BCR157
BCR157
法律資訊
BCR is a registered trademark of European Commission
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
A D Ayrton et al.
Biochemical pharmacology, 37(23), 4565-4571 (1988-12-01)
The ability of the aza-aromatic polycyclic aromatic hydrocarbons 10-azobenz(a)pyrene and benz(a)acridine to induce the rat hepatic microsomal mixed-function oxidases was compared to that of their non-heterocyclic analogues benz(a)pyrene and benz(a)anthracene respectively. All four hydrocarbons markedly increased the O-deethylations of ethoxyresorufin
N Motohashi et al.
Anticancer research, 12(1), 135-139 (1992-01-01)
Various synthetic derivatives of phenothiazines, benzo[a]phenothiazines and benz[c]acridines were compared for their abilities to induce antiplasmid activity against E. coli F'lac plasmid. Several phenothiazine derivatives were much more potent in antiplasmid activity than benzo[a]phenothiazine- or benz[c]acridine derivatives. Their antiplasmid activity
G R Southworth et al.
Archives of environmental contamination and toxicology, 10(5), 561-569 (1981-09-01)
The bioconcentration and metabolism of benz(a)acridine in fathead minnows (Pimephales promelas) was investigated using 14C-labelled benz(a)acridine. The rates of uptake, elimination, and metabolic transformation of benz(a)acridine were estimated in the fish. The equilibrium concentration factor [ratio of benz(a)acridine concentration in
N Motohashi et al.
Anticancer research, 12(4), 1207-1210 (1992-07-01)
The abilities of 14 phenothiazines, 8 benzo[a]phenothiazines and 12 benz[c]acridines to induce an antibacterial effect against Escherichia coli K12 were compared. Several phenothiazines, which showed antiplasmid activity, displayed the most potent antibacterial activity. All benz[c]acridine derivatives were moderately antibacterial, whereas
Benz[a]acridine.
IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans, 32, 123-127 (1983-12-01)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门
![二苯并[a,h]吖啶 BCR®, certified reference material](/deepweb/assets/sigmaaldrich/product/structures/383/751/70b25872-405f-49b1-b76b-ed5e018ce265/640/70b25872-405f-49b1-b76b-ed5e018ce265.png)
![苯并[h]喹啉 97%](/deepweb/assets/sigmaaldrich/product/structures/344/715/928932d2-4ca4-4402-b56c-85a80100ce17/640/928932d2-4ca4-4402-b56c-85a80100ce17.png)
![4H-benzo[def]carbazole AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/319/198/4208123a-456b-4f27-bc62-bc3af7c1d403/640/4208123a-456b-4f27-bc62-bc3af7c1d403.png)
![二苯并[a,i]吖啶 BCR®, certified reference material](/deepweb/assets/sigmaaldrich/product/structures/531/045/5d4c722d-9928-44fb-8bbb-7f4e27442f63/640/5d4c722d-9928-44fb-8bbb-7f4e27442f63.png)
![11H-Benzo[a]carbazole](/deepweb/assets/sigmaaldrich/product/structures/391/065/abfb4cba-81ab-44b8-a816-d8791a903400/640/abfb4cba-81ab-44b8-a816-d8791a903400.png)
![二苯并[c,h]吖啶 BCR®, certified reference material](/deepweb/assets/sigmaaldrich/product/structures/364/643/698df9fb-5b7d-467a-b47e-c8318e2ed298/640/698df9fb-5b7d-467a-b47e-c8318e2ed298.png)