推荐产品
等級
purum
品質等級
化驗
≥98.0% (GC)
形狀
liquid
折射率
n20/D 1.396 (lit.)
n20/D 1.396
bp
142 °C (lit.)
密度
0.885 g/mL at 25 °C (lit.)
SMILES 字串
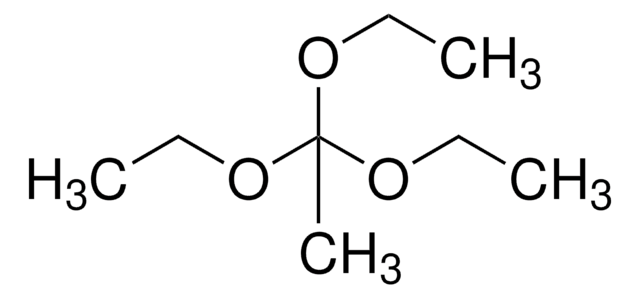
CCOC(C)(OCC)OCC
InChI
1S/C8H18O3/c1-5-9-8(4,10-6-2)11-7-3/h5-7H2,1-4H3
InChI 密鑰
NDQXKKFRNOPRDW-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
Triethyl orthoacetate is a general reagent used to functionalize alcohols with acetate groups. It can be used in following reactions:
- Stereocontrolled total synthesis of a naturally occuring indole alkaloid, (−)-aspidophytine.
- Conversion of allylic alcohols to γ,δ-unsaturated esters under mild acidic condition, a reaction popularly known as Johnson–Claisen rearrangement.
- Synthesis of heterocycles such as 2-oxazolines and quinazolin-4(3H)-one derivatives.
An efficient and versatile method for the synthesis of optically active 2-oxazolines: an acid-catalyzed condensation of ortho esters with amino alcohols.
Kamata K, et al.
The Journal of Organic Chemistry, 63(9), 113-3116 (1998)
Clay catalysis: condensation of orthoesters with O-substituted aminoaromatics into heterocycles.
Villemin D, et al.
Synthetic Communications, 26(15), 2895-2899 (1996)
Simple stereoselective version of the Claisen rearrangement leading to trans-trisubstituted olefinic bonds. Synthesis of squalene.
Johnson WS, et al.
Journal of the American Chemical Society, 92(3), 741-743 (1970)
Stereocontrolled total synthesis of (−)-aspidophytine.
Sumi S, et al.
Tetrahedron, 59(43), 8571-8587 (2003)
Reaction of orthoesters with alcohols in the presence of acidic catalysts: A study.
Kumar, HM et al.
Indian J. Chem. B, 44B(8), 1686-1692 (2005)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门