所有图片(4)
About This Item
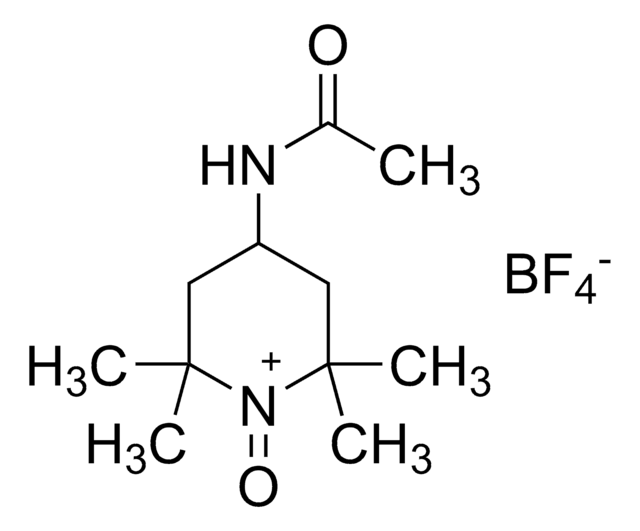
经验公式(希尔记法):
C11H21N2O2
CAS号:
分子量:
213.30
Beilstein:
3546225
EC號碼:
MDL號碼:
分類程式碼代碼:
12352005
PubChem物質ID:
NACRES:
NA.22
推荐产品
等級
purum
品質等級
化驗
≥98.0% (HPLC)
環保替代產品評分
old score: 9
new score: 8
Find out more about DOZN™ Scoring
環保替代產品特色
Waste Prevention
Safer Solvents and Auxiliaries
Inherently Safer Chemistry for Accident Prevention
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
143-145 °C (lit.)
143-146 °C
官能基
amide
環保替代類別
儲存溫度
2-8°C
SMILES 字串
CC(=O)NC1CC(C)(C)N([O])C(C)(C)C1
InChI
1S/C11H21N2O2/c1-8(14)12-9-6-10(2,3)13(15)11(4,5)7-9/h9H,6-7H2,1-5H3,(H,12,14)
InChI 密鑰
UXBLSWOMIHTQPH-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
4-Acetamido-2,2,6,6-tetramethylpiperidine 1-oxyl undergoes one-electron oxidation and reduction reactions. Reaction products were analyzed by 1H, 13C and 15N NMR spectral data.
We are committed to bringing you Greener Alternative Products, which adhere to one of the four categories of Greener Alternatives . This product belongs to category of Re-engineered products, showing key improvements in Green Chemistry Principles “Waste Prevention”, “Safer Solvents and Auxiliaries” and “Inherently Safer Chemistry for Accident Prevention”. Click here to view its DOZN scorecard.
應用
4-Acetamido-2,2,6,6-tetramethylpiperidine 1-oxyl radical may be employed in the catalytic system used for the regioselective oxidation of curdlan. It may be employed as model nitroxyl radical to study its reaction with thiophosgene. Reaction afforded 2,2,6,6-tetramethylpiperidine and 2,2,6,6-tetramethyl-1-hydroxypiperidine as major products.
其他說明
将醇轻度氧化成羰基的新型氧化剂
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
A 1H, 13C and 15N NMR study of oxidation and reduction products of 4-acetamido-2, 2, 6, 6-tetramethylpiperidine-1-oxyl.
Schilf W, et al.
Journal of Molecular Structure, 407(1), 1-3 (1997)
Reaction of Nitroxides with Sulfur Containing Compounds II [1]. Preparation of Nitroxides Bearing an Isothiocyanate Substituent in View of the Nitroxyl Group Reduction with Thiophosgene.
Zakrzewski J, et al.
Monatshefte fur Chemie / Chemical Monthly, 134(6), 843-850 (2003)
A Koceva-Chyła et al.
Anticancer research, 20(6B), 4611-4618 (2001-02-24)
The novel nitroxyl, Tempicol-3 (nitroxide-N-oxide) was synthesized and its capacity to act as a scavenger of hydroxyl radicals was tested. The concentration-dependent reducibility of this novel compound was also examined and compared with those of previously characterized nitroxides, Tempo and
Z. Ma et al.
The Journal of Organic Chemistry, 56, 6110-6110 (1991)
D Metodiewa et al.
Biochemistry and molecular biology international, 40(6), 1211-1219 (1996-12-01)
Two novel 2,2,6,6-tetramethylpiperidine derivatives (Tempace and Troxyl) were synthesized and their capacity to act as scavengers of superoxide, inhibitors of iron and ascorbate-driven Fenton reaction and radioprotectors was tested. The possibility for one-electron oxidation of novel compounds by heme-ferryl species
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门