推荐产品
應用
bioburden testing
cosmetics
pharmaceutical
water monitoring
相容性
for use with MILLIFLEX®
一般說明
Microbiological monitoring and testing in the pharmaceutical industry are highly regulated and a very complex field. In our long history of serving the pharmaceutical industry by pioneering and refining ground-breaking solutions, we have gained the regulatory and technical expertise to make compliance as simple as possible, and help save your valuable resources, by providing comprehensive range of professional and best-in-class services. Our Customer Validation Protocol makes validation faster, easier and ensures that your Milliflex Oasis® pump, accessories and related consumables do comply with the validated specifications. Our bioburden validation protocols follow international guidelines such as EP/USP and GMP.
With our best-in-class services you can:
With our best-in-class services you can:
- Optimize your QC lab workflow and ensure regulatory compliance
- Rely on comprehensive and ready-to-use validation packages
- Ensure the performance of your Milliflex Oasis® pump while reducing the risk of breakdown
應用
Our validation protocols are based on our internal product qualification test methods. These extensive protocols will enable the QC/QA lab to quickly initiate the validation master plan and perform IQ, OQ and PQ (suitability of the test methodology) with ease.
特點和優勢
Count on our comprehensive and ready-to-use validation protocols consisting of the following sections:
1. Validation Master Plan: Define structure, responsibilities for qualification
2. Installation Qualification (IQ)
4. Performance Qualification (PQ) Test Method suitability verification (microbiology validation procedures)
5. Final Report Summarizes all testing performed for final approval of validation
1. Validation Master Plan: Define structure, responsibilities for qualification
2. Installation Qualification (IQ)
- Verification and identification of with your Milliflex Oasis® pump, accessories and related consumables
- Verification of product′s utilities and operating environment requirements
- Equipment and personnel preparation
4. Performance Qualification (PQ) Test Method suitability verification (microbiology validation procedures)
5. Final Report Summarizes all testing performed for final approval of validation
法律資訊
MILLIFLEX is a registered trademark of Merck KGaA, Darmstadt, Germany
MILLIFLEX OASIS is a registered trademark of Merck KGaA, Darmstadt, Germany
商品
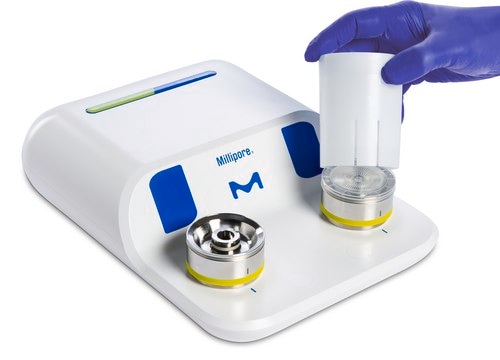
Milliflex Oasis® system monitors bioburden in various samples, increasing lab productivity.
Milliflex Oasis® system monitors bioburden in various samples, increasing lab productivity.
Milliflex Oasis® system monitors bioburden in various samples, increasing lab productivity.
Milliflex Oasis® system monitors bioburden in various samples, increasing lab productivity.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门