推荐产品
品質等級
化驗
≥97%
顏色
light yellow
mp
215 °C (dec.) (lit.)
儲存溫度
−20°C
SMILES 字串
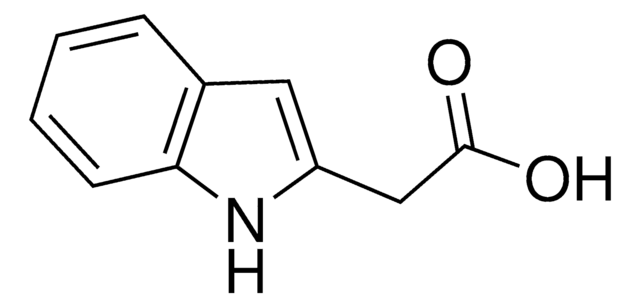
OC(=O)C(=O)Cc1c[nH]c2ccccc12
InChI
1S/C11H9NO3/c13-10(11(14)15)5-7-6-12-9-4-2-1-3-8(7)9/h1-4,6,12H,5H2,(H,14,15)
InChI 密鑰
RSTKLPZEZYGQPY-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
吲哚-3-丙酮酸可作为:
- 前体,由含血红素的酶合成色吡咯酸。
- Biginelli类支架合成中的反应物。
聯結
色氨酸的 α-酮类似物
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Direct formation of chromopyrrolic acid from indole-3-pyruvic acid by StaD, a novel hemoprotein in indolocarbazole biosynthesis
Asamizu, S, et al.
Tetrahedron Letters, 47(4), 473-475 (2006)
L Bacciottini et al.
Pharmacological research communications, 19(11), 803-817 (1987-11-01)
The effects of acute or repeated administration of indole-pyruvic acid (IPA), a keto-analogue of tryptophan (TRP), were studied in various brain areas of rats by measuring the changes of 5-hydroxytryptamine (5-HT) and of norepinephrine (NE) content and metabolism. The analgesic
Cyclic ketones and substituted α-keto acids as alternative substrates for novel Biginelli-like scaffold syntheses
Abelman, Matthew M, et al.
Tetrahedron Letters, 44(24), 4559-4562 (2003)
Goutam Chowdhury et al.
Chemical research in toxicology, 22(12), 1905-1912 (2009-10-29)
Aerobic incubation of the tryptophan transamination/oxidation product indole-3-pyruvic acid (I3P) at pH 7.4 and 37 degrees C yielded products with activity as Ah receptor (AHR) agonists. The extracts were fractionated using HPLC and screened for AHR agonist activity. Two compounds
Nathan D Tivendale et al.
Plant physiology, 159(3), 1055-1063 (2012-05-11)
Seeds of several agriculturally important legumes are rich sources of the only halogenated plant hormone, 4-chloroindole-3-acetic acid. However, the biosynthesis of this auxin is poorly understood. Here, we show that in pea (Pisum sativum) seeds, 4-chloroindole-3-acetic acid is synthesized via
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门