推荐产品
品質等級
化驗
99%
形狀
powder
mp
146-149 °C
147-149 °C (lit.)
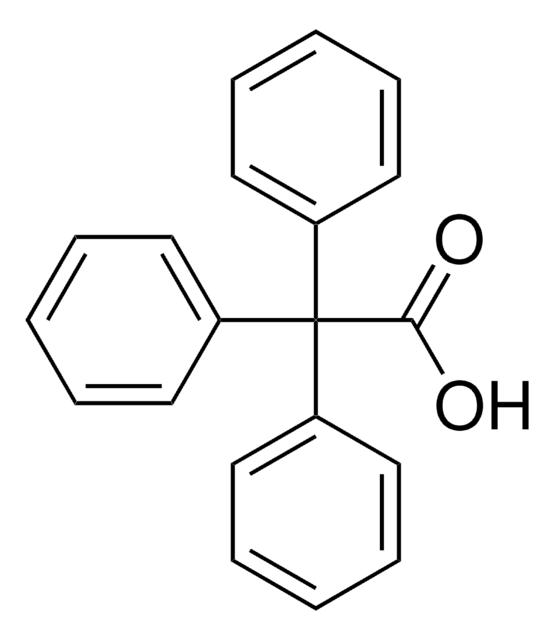
SMILES 字串
OC(=O)C(c1ccccc1)c2ccccc2
InChI
1S/C14H12O2/c15-14(16)13(11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10,13H,(H,15,16)
InChI 密鑰
PYHXGXCGESYPCW-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
二苯乙酸可作为试剂,用于在特戊酸酐和手性酰基转移催化剂存在的情况下通过不对称酯化进行外消旋 2-羟基γ-丁内酯和 1-杂芳基烷醇的动力学拆分。
还可用作:
还可用作:
- 添加剂,用于在钌催化剂存在的情况下以各种芳基卤化物进行 1-苯基β-咔啉的邻位芳基化。
- 催化剂,用于通过吖内酯和炔烃的铑催化偶联反应以及随后的氮杂-柯普重排合成2-烯丙基-3-噁唑啉-5-酮衍生物。
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
383.5 °F - closed cup
閃點(°C)
195.3 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Hugh D Glossop et al.
Biomacromolecules, 20(7), 2515-2529 (2019-05-31)
Peptides can serve as versatile therapeutics with a highly modular structure and tunable biophysical properties. In particular, the efficacy of peptide antibiotics against drug-resistant pathogens is of great promise, as few new classes of antibiotics are being developed to overcome
Jinqiang Kuang et al.
Angewandte Chemie (International ed. in English), 56(29), 8422-8425 (2017-05-18)
Rhodium-catalyzed regioselective addition of azlactones to internal alkynes combined with aza-Cope rearrangement provides efficient atom economic access to 2-allyl-3-oxazolin-5-one derivatives. Extension to a triple domino process, in which the above process is combined with in situ azlactone formation starting from amino
Kinetic Resolution of Racemic 1-Heteroarylalkanols by Asymmetric Esterification Using Diphenylacetic Acid with Pivalic Anhydride and a Chiral Acyl-transfer Catalyst
Shiina I, et al.
Chemistry Letters (Jpn), 40(2), 147-149 (2011)
Subramani Rajkumar et al.
The Journal of organic chemistry, 80(11), 5532-5545 (2015-04-11)
A Ru(II)-catalyzed C-H arylation approach has been developed utilizing β-carboline alkaloids as the directing group. Selective formations of diarylated products from moderate to excellent yields were accomplished. Broad substrate scope with excellent functional group tolerance for C1-phenyl/thienyl/PAHs-β-carbolines was demonstrated. X-ray
Kenya Nakata et al.
Organic letters, 15(6), 1170-1173 (2013-03-07)
Various optically active 2-hydroxy-γ-butyrolactone derivatives are produced via the kinetic resolution of racemic 2-hydroxy-γ-butyrolactones with diphenylacetic acid using pivalic anhydride and (R)-benzotetramisole ((R)-BTM), a chiral acyl-transfer catalyst. Importantly, the substrate scope of this novel protocol is fairly broad (12 examples
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门