推荐产品
形狀
powder
品質等級
反應適用性
reagent type: oxidant
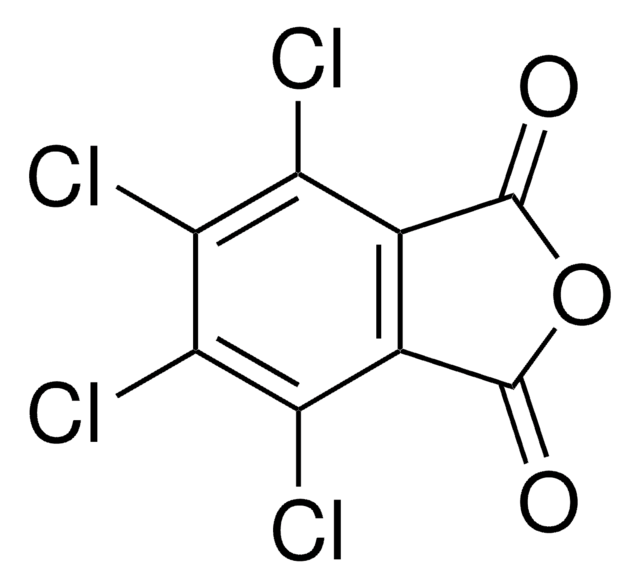
SMILES 字串
O=C1N(O)C(C2=C(Cl)C(Cl)=C(Cl)C(Cl)=C21)=O
InChI
1S/C8HCl4NO3/c9-3-1-2(4(10)6(12)5(3)11)8(15)13(16)7(1)14/h16H
InChI 密鑰
UTRBHXSKVVPTLY-UHFFFAOYSA-N
一般說明
應用
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
从最新的版本中选择一种:
分析证书(COA)
其他客户在看
商品
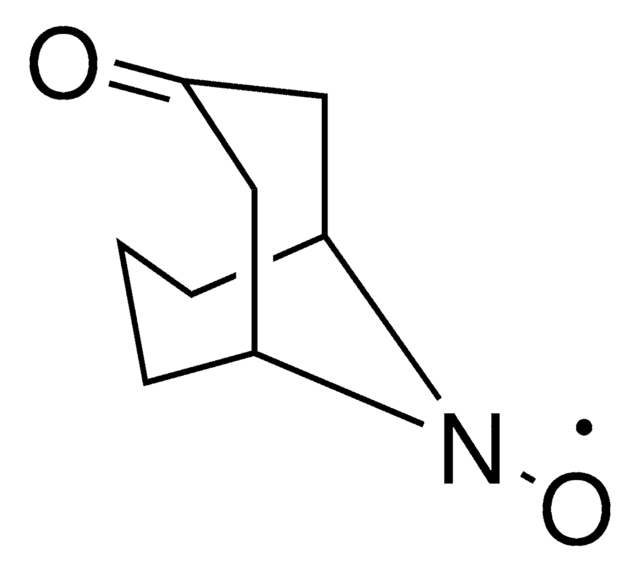
Phil Baran develops ALD00564 reagent as a safe, cost-effective alternative for allylic oxidations and cross-coupling reactions.
Phil Baran develops ALD00564 reagent as a safe, cost-effective alternative for allylic oxidations and cross-coupling reactions.
Phil Baran develops ALD00564 reagent as a safe, cost-effective alternative for allylic oxidations and cross-coupling reactions.
Phil Baran develops ALD00564 reagent as a safe, cost-effective alternative for allylic oxidations and cross-coupling reactions.
相关内容
The Baran Group works with Sigma-Aldrich in providing a portfolio of zinc-based reagents promoting difluoromethylation, trifluoromethylation, trifluoroethylation and isopropylation of aryl and heteroaryl motifs. Baran’s lab has also helped introduce a portable desaturase (Tz0Cl), which promotes the installation of alcohol and amine groups and leaves behind a highly useful tosyl group for further transformations.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门