推荐产品
质量水平
方案
95%
表单
solid
mp
164-169 °C
官能团
ether
phenyl
SMILES字符串
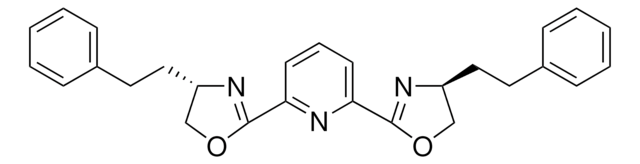
CC(C1=N[C@H](C2=CC=CC=C2)[C@H](C3=CC=CC=C3)O1)(C)C4=N[C@H](C5=CC=CC=C5)[C@H](C6=CC=CC=C6)O4
InChI
1S/C33H30N2O2/c1-33(2,31-34-27(23-15-7-3-8-16-23)29(36-31)25-19-11-5-12-20-25)32-35-28(24-17-9-4-10-18-24)30(37-32)26-21-13-6-14-22-26/h3-22,27-30H,1-2H3/t27-,28-,29+,30+/m1/s1
InChI key
ZWWGNCSTEMMQOQ-XAZDILKDSA-N
应用
(4R,4′R,5S,5′S)-2,2′-(1-Methylethylidene) or (4S,5R)-Bis-Phbox) can be used as a ligand:
This chiral Box ligand was most recently shown to mediate an asymmetric aminofluorination of olefins utlizing Xtalfuor-E (719439) and TREAT-HF (344648). The resulting cyclic carbamates can be readily converted into their concomitant beta-fluoro amino acids.
- To prepare selective exo-catalysts for enantioselective 1,3-dipolar cycloaddition reactions.
- In the asymmetric aminooxygenation of alkenes in the presence of tetramethylaminopyridyl radical (TEMPO) as an oxidant and copper(II) triflate as a catalyst.
- In asymmetric aminofluorination of olefins using an iron catalyst.
This chiral Box ligand was most recently shown to mediate an asymmetric aminofluorination of olefins utlizing Xtalfuor-E (719439) and TREAT-HF (344648). The resulting cyclic carbamates can be readily converted into their concomitant beta-fluoro amino acids.
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
其他客户在看
Copper catalyzed enantioselective intramolecular aminooxygenation of alkenes
Fuller PH, et al.
Journal of the American Chemical Society, 130(52), 17638-17639 (2008)
Iron (II)-catalyzed intramolecular olefin aminofluorination
Lu Deng-Fu, et al.
Organic Letters, 16(11), 2912-2915 (2014)
In Search of exo-Selective Catalysts for Enantioselective 1, 3-Dipolar Cycloaddition between Acryloyloxazolidinone and Diphenylnitrone
Desimoni G, et al.
European Journal of Organic Chemistry, 2005(6), 1020-1027 (2005)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门
![2,6-双[(3aR,8aS)-(+)-8H-茚并[1,2-d]噁唑啉-2-基)吡啶 ≥94%](/deepweb/assets/sigmaaldrich/product/structures/123/619/565288e2-e1c9-4825-a440-17e786bc2c27/640/565288e2-e1c9-4825-a440-17e786bc2c27.png)
![(-)-2,2′-异亚丙基双[(4S)-4-苯基-2-噁唑啉] 97%](/deepweb/assets/sigmaaldrich/product/structures/297/720/a29f61c3-34e4-410c-acdd-241699b80af3/640/a29f61c3-34e4-410c-acdd-241699b80af3.png)
![2,2′-异亚丙基双[(4S)-4-叔丁基-2-噁唑啉] 99%](/deepweb/assets/sigmaaldrich/product/structures/334/357/19788a81-5365-46fd-978b-6b98382b1117/640/19788a81-5365-46fd-978b-6b98382b1117.png)
![2,6-双[(4S)-4-苯基-2-噁唑啉基]吡啶 98%](/deepweb/assets/sigmaaldrich/product/structures/372/262/fb5c79fe-8277-48b0-a73e-4124c7c2c41c/640/fb5c79fe-8277-48b0-a73e-4124c7c2c41c.png)
![2,2′-双[(4S)-4-苄基-2-噁唑啉] 98%](/deepweb/assets/sigmaaldrich/product/structures/139/783/42da3c77-52af-401b-8525-35d96415e284/640/42da3c77-52af-401b-8525-35d96415e284.png)
![(+)-2,2′-异亚丙基双[(4R)-4-苯基-2-噁唑啉] 97%](/deepweb/assets/sigmaaldrich/product/structures/232/241/07f8baaa-0ba2-49e0-8ac2-f6d256fb2c84/640/07f8baaa-0ba2-49e0-8ac2-f6d256fb2c84.png)
![[3aR-[2(3′aR*,8′aS*),3′aβ,8′aβ]]-(+)-2,2′-亚甲基双[3a,8a-二氢-8H-茚并[1,2-]噁唑] 98%](/deepweb/assets/sigmaaldrich/product/structures/134/031/294d2464-1571-4514-8e4c-c0cda1c1df7b/640/294d2464-1571-4514-8e4c-c0cda1c1df7b.png)
![2,2′-亚甲基双[(4S)-4-苯基-2-噁唑啉] 97%](/deepweb/assets/sigmaaldrich/product/structures/255/350/4403d4f8-c973-4da7-a5b6-2e93d1eacb10/640/4403d4f8-c973-4da7-a5b6-2e93d1eacb10.png)
![2,6-双[(4R)-(+)-异丙基-2-噁唑啉-2-基]吡啶 99%](/deepweb/assets/sigmaaldrich/product/structures/349/609/8673c46e-368a-47a6-a9bd-52bbe13d490a/640/8673c46e-368a-47a6-a9bd-52bbe13d490a.png)

![(4S)-(+)-苯基-α-[(4S)-苯基噁唑烷-2-亚基]-2-噁唑啉-2-乙腈 97%](/deepweb/assets/sigmaaldrich/product/structures/117/759/d4e6e882-8577-4dcf-84fe-506633ae811a/640/d4e6e882-8577-4dcf-84fe-506633ae811a.png)
![2,2′-亚甲基双[6-(2H-苯并三唑2基)-4-(1,1,3,3-四甲基丁基)苯酚] 99%](/deepweb/assets/sigmaaldrich/product/structures/236/824/ce89085c-b9e1-4ea0-8157-44b6f9466ed6/640/ce89085c-b9e1-4ea0-8157-44b6f9466ed6.png)