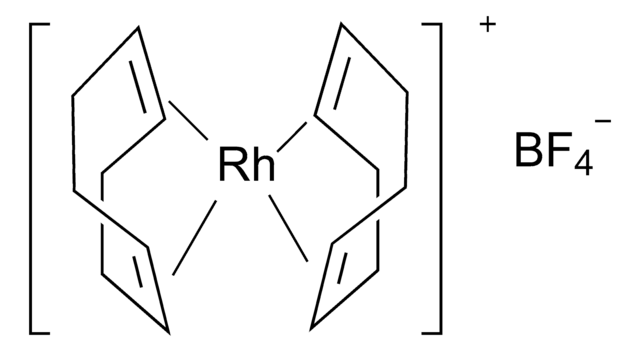
推荐产品
形狀
solid
品質等級
反應適用性
core: rhodium
reagent type: catalyst
mp
155-156 °C (D)
儲存溫度
−20°C
SMILES 字串
[Rh+].C1CC=CCCC=C1.C2CC=CCCC=C2.FC(F)(F)c3cc(cc(c3)C(F)(F)F)[B-](c4cc(cc(c4)C(F)(F)F)C(F)(F)F)(c5cc(cc(c5)C(F)(F)F)C(F)(F)F)c6cc(cc(c6)C(F)(F)F)C(F)(F)F
InChI
1S/C32H12BF24.2C8H12.Rh/c34-25(35,36)13-1-14(26(37,38)39)6-21(5-13)33(22-7-15(27(40,41)42)2-16(8-22)28(43,44)45,23-9-17(29(46,47)48)3-18(10-23)30(49,50)51)24-11-19(31(52,53)54)4-20(12-24)32(55,56)57;2*1-2-4-6-8-7-5-3-1;/h1-12H;2*1-2,7-8H,3-6H2;/q-1;;;+1/b;2*2-1-,8-7-;
InChI 密鑰
HQQAEXNVAYMZON-AUUWQFPRSA-N
相关类别
應用
Bis(1,5-cyclooctadiene)rhodium(I) tetrakis[bis(3,5-trifluoromethyl)phenyl]borate can be used as a catalyst for various transformations, including hydrogenation, intramolecular C-H alkenylation, cycloaddition of enynes, and reductive coupling reactions.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Intramolecular C- H Alkenylation of N-Alkynylindoles: Exo and Endo Selective Cyclization According to the Choice of Metal Catalyst
Shibata, T, et al.
Advanced Synthesis & Catalysis, 359(11), 1849-1853 (2017)
Highly diastereo-and enantioselective construction of both central and axial chiralities by Rh-catalyzed [2+ 2+ 2] cycloaddition
Shibata T, et al.
Organic & Biomolecular Chemistry, 6(23), 4296-4298 (2008)
Proline-Based P, O Ligand/Iridium Complexes as Highly Selective Catalysts: Asymmetric Hydrogenation of Trisubstituted Alkenes
Rageot D, et al.
Angewandte Chemie (International Edition in English), 123(41), 9772-9775 (2011)
Intramolecular C- H Alkenylation of N-Alkynylindoles: Exo and Endo Selective Cyclization According to the Choice of Metal Catalyst
Shibata, T, et al.
advanced synthesis and catalysis, 359(11), 1849-1853 (2017)
Venukrishnan Komanduri et al.
Journal of the American Chemical Society, 130(38), 12592-12593 (2008-09-02)
Hydrogenation of 2-vinyl azines 1a-1e in the presence of N-arylsulfonyl imines 2a-2l at ambient temperature and pressure employing cationic rhodium catalysts ligated by tri-2-furylphosphine results in regioselective reductive coupling to furnish branched products of imine addition 3a-3v, which embody modest
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门