所有图片(2)
About This Item
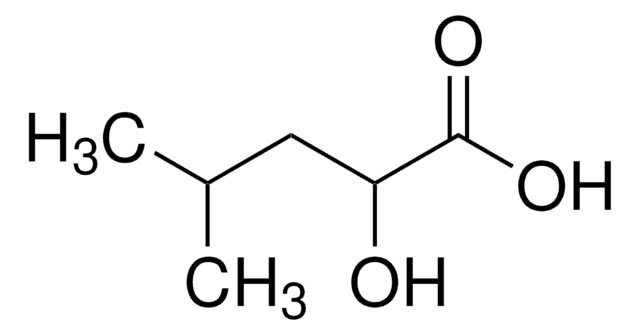
经验公式(希尔记法):
C5H10O3
CAS号:
分子量:
118.13
Beilstein:
1721139
MDL號碼:
分類程式碼代碼:
51113400
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
≥98.0% (T)
形狀
solid
光學活性
[α]20/D −17±1°, c = 1% in chloroform
mp
64-67 °C
SMILES 字串
CC(C)[C@@H](O)C(O)=O
InChI
1S/C5H10O3/c1-3(2)4(6)5(7)8/h3-4,6H,1-2H3,(H,7,8)/t4-/m1/s1
InChI 密鑰
NGEWQZIDQIYUNV-SCSAIBSYSA-N
應用
D-α-Hydroxyisovaleric acid may be used in the preparation of biodegradable, optically active and isotactic poly(D-2-hydroxy-3-methylbutanoic acid).
其他說明
该手性 α-羟基酸用于合成肽和缩酚酸肽。 还可用作手性结构单元。 通过二氧戊环酮中间体合成 α-取代 α-羟基酸。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
从最新的版本中选择一种:
分析证书(COA)
Lot/Batch Number
其他客户在看
R L Johnson
Journal of medicinal chemistry, 23(6), 666-669 (1980-06-01)
The following N-(alpha-hydroxylakanoyl) derivatives of Leu-Val-Phe-OCH3 were synthesized and tested for their ability to inhibit human amniotic renin: D- and L-alpha-hydroxyisocaproyl-Leu-Val-Phe-OCH3, D- and L-alpha-hydroxyisovaleryl-Leu-Val-Phe-OCH3, L-2-hydroxy-3-phenylpropanoyl-Leu-Val-Phe-OCH3, and D- and L-alpha-hydroxyphenylacetyl-Leu-Val-Phe-OCH3. Analysis of the compounds through the use of Dixion plots showed
H.-O. Kim et al.
The Journal of Organic Chemistry, 52, 4531-4531 (1987)
W. Hartwig et al.
Liebigs Ann. Chem., 1952-1952 (1982)
Hetero-stereocomplex formation between substituted poly (lactic acid) s with linear and branched side chains, poly (l-2-hydroxybutanoic acid) and poly (d-2-hydroxy-3-methylbutanoic acid).
Tsuji H and Hayakawa T.
Polymer, 55(3), 721-726 (2014)
Hye-Ran Yoon
Archives of pharmacal research, 30(3), 387-395 (2007-04-12)
A rapid dried-filter paper plasma-spot analytical method was developed to quantify organic acids, amino acids, and glycines simultaneously in a two-step derivatization procedure with good sensitivity and specificity. The new method involves a two-step trimethylsilyl (TMS) - trifluoroacyl (TFA) derivatization
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门