所有图片(1)
About This Item
经验公式(希尔记法):
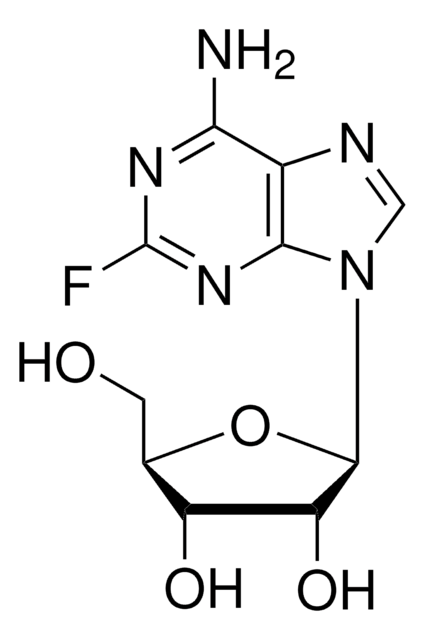
C5H4FN5
CAS号:
分子量:
153.12
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
96%
mp
>350 °C (lit.)
SMILES 字串
Nc1[nH]c(F)nc2ncnc12
InChI
1S/C5H4FN5/c6-5-10-3(7)2-4(11-5)9-1-8-2/h1H,(H3,7,8,9,10,11)
InChI 密鑰
WKMPTBDYDNUJLF-UHFFFAOYSA-N
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
P Huang et al.
Biochemical pharmacology, 36(18), 2945-2950 (1987-09-15)
2-Fluoroadenine (F-Ade) is a metabolite of 9-beta-D-arabinofuranosyl-2-fluoroadenine (F-ara-A) that may be involved in the development of toxic side effects from this anticancer drug. The liberation of F-Ade from F-ara-A has been examined in different biological systems. Extracts of Escherichia coli
Yukio Kitade et al.
Nucleic acids research. Supplement (2001), (3)(3), 5-6 (2003-09-27)
Carbocyclic and acyclic nucleosides possessing 2-fluoroadenine, such as 2-fluoronoraristeromycin (6) and 2-fluoro-9-[(2S,3R)-2,3,4-trihydroxy-butyl-1-yl]adenine (8), were synthesized and their inhibitory activities against human and Plasmodium falciparum recombinant SAH hydrolase were investigated.
D Voeks et al.
Gene therapy, 9(12), 759-768 (2002-06-01)
A gene-directed enzyme pro-drug therapy (GDEPT) based on purine nucleoside phosphorylase (PNP), that converts the prodrug, fludarabine to 2-fluoroadenine, has been described, but studies are limited compared with other GDEPTs. We investigated the in vitro and in vivo efficacies of
V I Avramis et al.
Biochemical and biophysical research communications, 113(1), 35-43 (1983-05-31)
Murine P388 cells incubated in vitro with the anticancer drug arabinosyl 2-fluoroadenine accumulate its 5'-triphosphate, F-araATP, as the major phosphorylated metabolite. A new chromatographically separate metabolite that accumulated to levels 10% of that of F-araATP was identified as 2-fluoro-ATP, by
Sepideh Afshar et al.
Protein science : a publication of the Protein Society, 18(5), 1107-1114 (2009-04-24)
A double mutant of human purine nucleoside phosphorylase (hDM) with the amino acid mutations Glu201Gln:Asn243Asp cleaves adenosine-based prodrugs to their corresponding cytotoxic drugs. When fused to an anti-tumor targeting component, hDM is targeted to tumor cells, where it effectively catalyzes
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门