About This Item
推荐产品
品質等級
化驗
98%
光學活性
[α]20/D −120.9°, c = 1 in chloroform
環保替代產品特色
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
102-104 °C (lit.)
環保替代類別
, Aligned
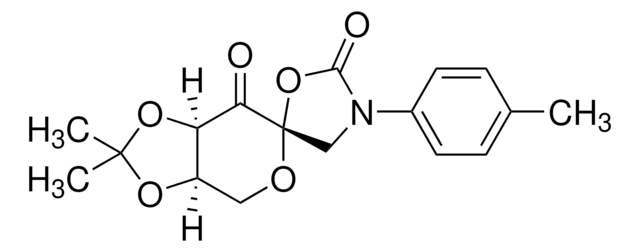
SMILES 字串
CC1(C)O[C@@H]2CO[C@]3(COC(C)(C)O3)C(=O)[C@@H]2O1
InChI
1S/C12H18O6/c1-10(2)15-6-12(18-10)9(13)8-7(5-14-12)16-11(3,4)17-8/h7-8H,5-6H2,1-4H3/t7-,8-,12+/m1/s1
InChI 密鑰
IVWWFWFVSWOTLP-RWYTXXIDSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
An Efficient Catalytic Asymmetric Epoxidation Method
Use of an Iridium-Catalyzed Redox-Neutral Alcohol-Amine Coupling on Kilogram Scale for the Synthesis of a GlyT1 Inhibitor
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
商品
Catalytic asymmetric epoxidation of alkenes has been the focus of many research efforts over the past two decades, the best known methods probably being those developed by Sharpless and Jacobsen-Katsuki.
Catalytic asymmetric epoxidation of alkenes has been the focus of many research efforts over the past two decades, the best known methods probably being those developed by Sharpless and Jacobsen-Katsuki.
Catalytic asymmetric epoxidation of alkenes has been the focus of many research efforts over the past two decades, the best known methods probably being those developed by Sharpless and Jacobsen-Katsuki.
Catalytic asymmetric epoxidation of alkenes has been the focus of many research efforts over the past two decades, the best known methods probably being those developed by Sharpless and Jacobsen-Katsuki.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门