推荐产品
交聯
1 % cross-linked
反應適用性
reaction type: Fmoc solid-phase peptide synthesis
reactivity: alcohol reactive
標籤範圍
~0.7 mmol/g loading
SMILES 字串
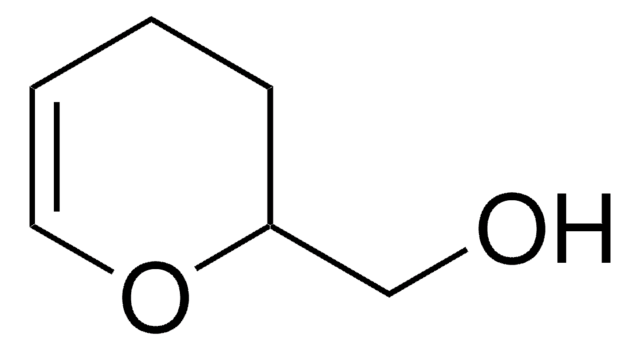
C=Cc1ccccc1.C=Cc2ccc(C=C)cc2.C3CC(COCc4ccccc4)OC=C3
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
相关内容
The Ellman group has participated in the development of a variety of C-H functionalization methods. An electron rich phosphine ligand has proven to be very useful for a variety of Rh(I)-catalyzed C-C bond forming reactions applicable to heterocycle synthesis as exemplified in the recent Science paper “Proton Donor Acidity Controls Selectivity in Nonaromatic Nitrogen Heterocycle Synthesis.” Another useful ligand developed for the highly functional group compatible direct arylation of nitrogen heterocycles is described in a 2008 J. Am. Chem. Soc. paper “Rh(I)-Catalyzed Arylation of Heterocycles via C-H Bond Activation: Expanded Scope through Mechanistic Insight.” The Ellman group also developed the chiral amine reagent tert-Butanesulfinamide, which is extensively used in academics and industry for the asymmetric synthesis of amines. A comprehensive survey of tert-Butanesulfinamide methods and applications up through 2009 is provided in the 2010 Chemical Reviews article, “Synthesis and Applications of tert-Butanesulfinamide.”
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门



![2- {2- [2-(2-巯基乙氧基)乙氧基]乙氧基}乙醇 97%](/deepweb/assets/sigmaaldrich/product/structures/130/969/7d2ec2b4-e0f1-4836-aeb2-139994173612/640/7d2ec2b4-e0f1-4836-aeb2-139994173612.png)


![1,4-Bis[4-(11-acryloyloxyundecyloxy)benzoyloxy]-2-methylbenzene](/deepweb/assets/sigmaaldrich/product/structures/215/829/62728d6f-7668-4693-94e2-37abc69d1144/640/62728d6f-7668-4693-94e2-37abc69d1144.png)
