495492
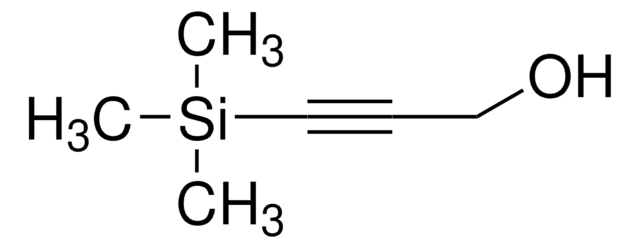
叔丁基二甲基(2-丙炔氧基)硅烷
97%
别名:
(1,1-Dimethylethyl)dimethyl(2-propyn-1-yloxy)silane, 1-(tert-Butyldimethylsilyloxy)-2-propyne, 3-(Dimethyl-tert-butylsiloxy)propyne, 3-(tert-Butyldimethylsilyloxy)-1-propyne, 3-(tert-Butyldimethylsilyloxy)propyne, 3-tert-Butyldimethylsiloxy-1-propyne, Dimethyl(2-Propynyloxy)(tert-butyl)silane
About This Item
推荐产品
品質等級
化驗
97%
折射率
n20/D 1.429 (lit.)
bp
40 °C/8 mmHg (lit.)
密度
0.84 g/mL at 25 °C (lit.)
SMILES 字串
CC(C)(C)[Si](C)(C)OCC#C
InChI
1S/C9H18OSi/c1-7-8-10-11(5,6)9(2,3)4/h1H,8H2,2-6H3
InChI 密鑰
ZYDKYFIXEYSNPO-UHFFFAOYSA-N
一般說明
應用
其他客户在看
商品
Alkynes' versatility enables reactions like addition, metathesis, hydroboration, cleavage, coupling, and cycloadditions in synthetic chemistry.
Alkynes' versatility enables reactions like addition, metathesis, hydroboration, cleavage, coupling, and cycloadditions in synthetic chemistry.
Alkynes' versatility enables reactions like addition, metathesis, hydroboration, cleavage, coupling, and cycloadditions in synthetic chemistry.
Alkynes' versatility enables reactions like addition, metathesis, hydroboration, cleavage, coupling, and cycloadditions in synthetic chemistry.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门