推荐产品
品質等級
化驗
98%
形狀
solid
mp
95-97 °C (lit.)
儲存溫度
2-8°C
SMILES 字串
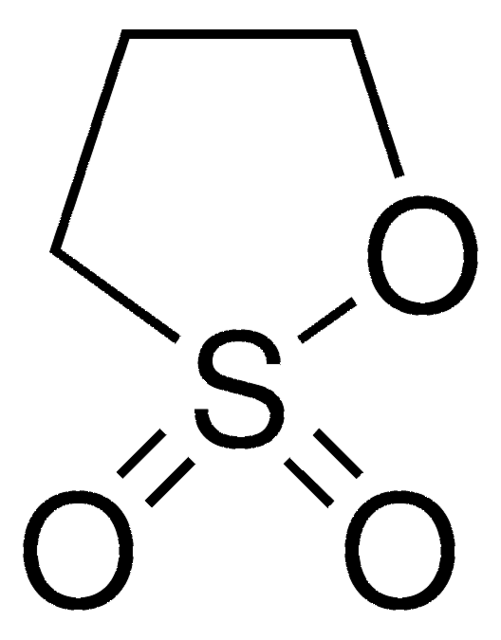
O=S1(=O)OCCO1
InChI
1S/C2H4O4S/c3-7(4)5-1-2-6-7/h1-2H2
InChI 密鑰
ZPFAVCIQZKRBGF-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
1,3,2-Dioxathiolane 2,2-dioxide, also known as Ethylene sulfate, is a cyclic sulfate that is commonly used as an alkylating agents and intermediate in the synthesis of disulfates and sulfonate surfactants.
應用
1,3,2-二氧杂环戊烷-2,2-二氧化物可用于制备咪唑烷鎓盐。
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1B
儲存類別代碼
8B - Non-combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Novel, one-pot procedure for the synthesis of 2-arylethanol derivatives
Torsten S, et al.
Synthesis, 2008, 1793-1797 (2008)
Synthesis and kinetic study of 1, 3, 2-dioxathiolane 2, 2-dioxide in microreactors
Ting W, et al.
Reaction Chemistry & Engineering (2024)
1, 3, 2-Dioxathiolane 2, 2-Dioxide
Nicholas L J, et al.
e-EROS Encyclopedia of Reagents for Organic Synthesis (2001)
Synthesis of hydroxy sulfonate surfactants
Oystein R, et al.
Molecules (Basel), 10, 1169-1178 (2005)
Rodolphe Jazzar et al.
Journal of organometallic chemistry, 691(14), 3201-3205 (2006-01-01)
Protonated versions of N-heterocyclic carbenes (NHC,H(+)) are classically prepared by closing the ring through the introduction of the CH+ fragment. He we report a totally different synthetic approach, which can be viewed as the addition of a 1,3-diazaallyl anion to
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门