推荐产品
化驗
97%
反應適用性
reaction type: C-C Bond Formation
折射率
n20/D 1.513 (lit.)
bp
192-193 °C/11 mmHg (lit.)
密度
1.179 g/mL at 25 °C (lit.)
官能基
ketone
phenyl
phosphonate
SMILES 字串
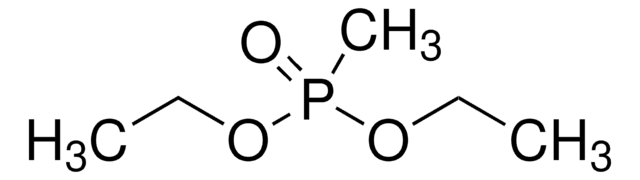
CCOP(=O)(CC(=O)c1ccccc1)OCC
InChI
1S/C12H17O4P/c1-3-15-17(14,16-4-2)10-12(13)11-8-6-5-7-9-11/h5-9H,3-4,10H2,1-2H3
InChI 密鑰
HPEVTTNSIPGLEL-UHFFFAOYSA-N
應用
Reactant involved in:
- Asymmetric Michael addition of β-oxo phosphonates to nitro olefins
- Gem-chlorofluorination of keto phosphonates with subsequent functionalization of the products
- Cyclocondensation reactions to produce arylphosphonates
- Diazo transfer reactions for synthesis of diazo-phosphonyl compounds
- Horner-Wadsworth-Emmons reactions
- Inverse-electron-demand Diels-Alder reactions
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves
从最新的版本中选择一种:
分析证书(COA)
Lot/Batch Number
Douglass F Taber et al.
Tetrahedron letters, 49(48), 6904-6906 (2009-12-01)
An convenient reagent for the one-carbon homologation of an aldehyde to the corresponding alkyne is reported. This reagent allows this conversion to conveniently be carried out on a large scale under ambient conditions.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门