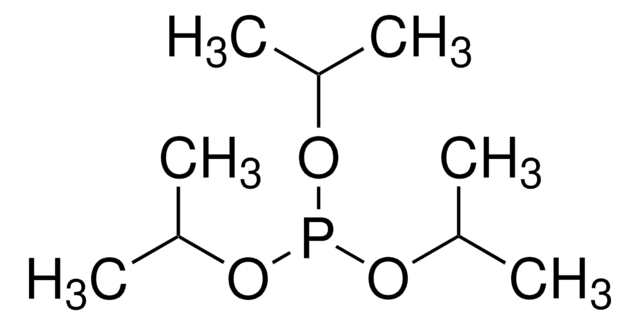
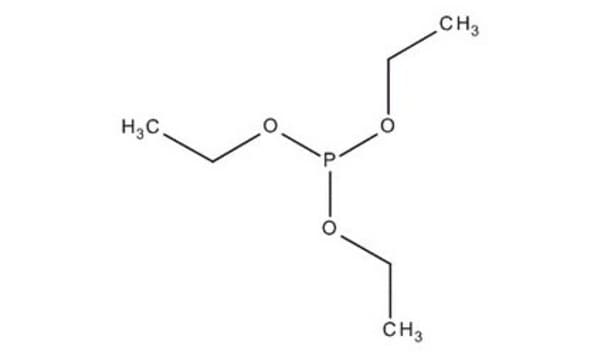
推荐产品
應用
Used as a reducing agent; can react with electrophiles to form phosphonates or phosphates; forms a stable complex with copper(I) iodide.
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Aquatic Chronic 3 - Flam. Liq. 3 - Skin Sens. 1B
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 2
閃點(°F)
129.2 °F - closed cup
閃點(°C)
54 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Kyriacos Agapiou et al.
Journal of the American Chemical Society, 126(14), 4528-4529 (2004-04-09)
Exposure of enone substrates 1a-18a, which possess appendant ketone, ester, and nitrile moieties, to Et2Zn in the presence of catalytic Cu(OTf)2/P(OEt)3 provides the cyclized products in good to excellent yields and diastereoselectivities. These results represent the first use of ketones
Stowell, J. K.; Widlanski, T. S. et al.
Tetrahedron Letters, 36, 1825-1825 (1995)
Gabriele Albertin et al.
Dalton transactions (Cambridge, England : 2003), 44(35), 15470-15480 (2015-08-04)
Diazoalkane complexes [Ru(Tp)(N2CAr1Ar2)(PPh3)L]BPh4 ( and ) [Tp = tris(pyrazolyl)borate; L = P(OMe)3, P(OEt)3; Ar1 = Ar2 = Ph; Ar1 = Ph, Ar2 = p-tolyl; Ar1Ar2 = C12H8] were prepared by allowing chloro-compounds RuCl(Tp)(PPh3)L to react with diazoalkane in the presence
Misner, J. W.; Fisher, J. W. et al.
Tetrahedron Letters, 44, 5991-5991 (2003)
Rocky J Barney et al.
The Journal of organic chemistry, 76(8), 2875-2879 (2011-03-17)
Benzyl phosphonate esters often serve as reagents in Horner-Wadsworth-Emmons reactions. In most cases, they can be prepared from benzylic alcohols via formation of the corresponding halide followed by an Arbuzov reaction. To identify a more direct synthesis of phosphonate esters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门