所有图片(1)
About This Item
经验公式(希尔记法):
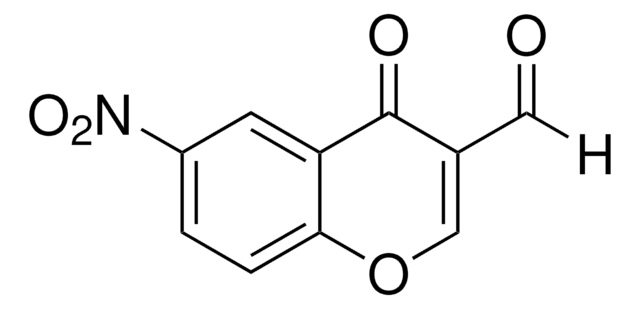
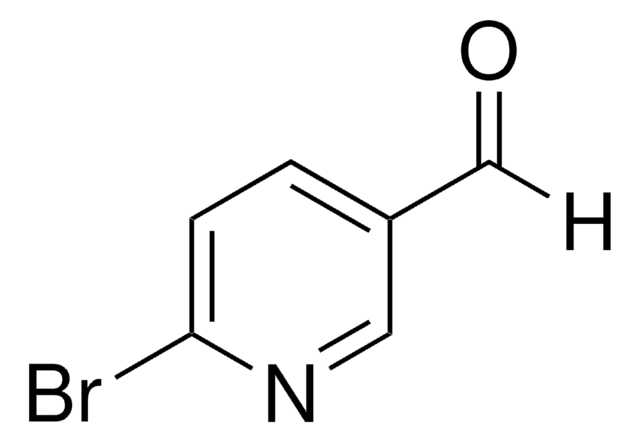
C10H5BrO3
CAS号:
分子量:
253.05
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
99%
mp
190-193 °C (lit.)
SMILES 字串
[H]C(=O)C1=COc2ccc(Br)cc2C1=O
InChI
1S/C10H5BrO3/c11-7-1-2-9-8(3-7)10(13)6(4-12)5-14-9/h1-5H
InChI 密鑰
PCEZXSJBHMOQFT-UHFFFAOYSA-N
基因資訊
human ... PTPN1(5770)
一般說明
6-Bromo-3-formylchromone (6-Bromo-4-oxo-4H-1-benzopyran-3-carboxaldehyde) is a 3-formylchromone derivative. In vivo cytotoxic activity of 6-bromo-3-formylchromone against normal and tumor cells has been tested.
應用
6-Bromo-3-formylchromone is the suitable reagent used in a study to investigate the multidrug resistance reversal by some 3- formylchromones in human colon cancer and mouse lymphoma cells transfected with the human MDR1 gene. It may be used in the preparation of chromone containing sulfonamides.
6-Bromo-3-formylchromone may be used in the preparation of 6′-bromopyranothiazine-4,7-diones.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
A study of the reactions of 2-aryl-4-hydroxy-6H-1,3-thiazin-6-ones with chromone-3-carboxaldehydes.
Shutov RV, et al.
Tetrahedron Letters, 52(2), 266-269 (2011)
Farukh Arjmand et al.
Bioorganic chemistry, 104, 104327-104327 (2020-11-05)
Copper-based antitumor drug entities 1-3 derived from substituted (F-, Br-, -CH3) 3-formylchromone pharmacophore were synthesized and thoroughly characterized by spectroscopic and single X-ray crystallographic studies. These complexes show structural novelty due to presence of the X-bonds in chromone scaffold which
Mariya al-Rashida et al.
Bioorganic & medicinal chemistry, 19(11), 3367-3371 (2011-05-11)
Series of chromone containing sulfonamides were prepared by the reaction of (un)substituted 3-formylchromones with 3-aminobenzenesulfonamide and 4-aminobenzenesulfonamide. Bovine carbonic anhydrase (bCA) inhibitory activity of these newly synthesized compounds was determined. All compounds were active and possessed excellent bCA inhibitory activities
Masami Kawase et al.
In vivo (Athens, Greece), 21(5), 829-834 (2007-11-21)
Several 3-formylchromone derivatives were examined for their tumor cell-cytotoxic, anti-Helicobacter pylori, urease inhibitory and anti-HIV activity. Comparing their relative cytotoxicity against four human tumor cell lines and three normal human cells, tumor cell-specific cytotoxicity was detected in some 3-formylchromone derivatives.
Zoltán Baráth et al.
In vivo (Athens, Greece), 20(5), 645-649 (2006-11-10)
Several new 3-formylchromone derivatives proved to be modifiers of multidrug resistance in mouse lymphoma cells and in human Colo320 colon cancer cells. There is apparently a structure-activity relationship between the antiproliferative multidrug resistance-reversing effect and the chemical structure of the
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门