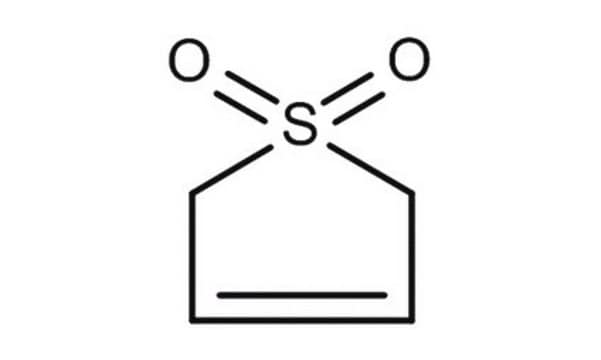
推荐产品
蒸汽密度
1.9 (15 °C, vs air)
质量水平
蒸汽压
1863 mmHg ( 21 °C)
方案
≥99%
自燃温度
788 °F
包含
p-tert-butylcatechol as inhibitor
expl. lim.
12 %
沸点
−4.5 °C (lit.)
mp
−109 °C (lit.)
溶解性
water: soluble 0.5 g/L at 20 °C
密度
0.62 g/mL at 20 °C (lit.)
储存温度
2-8°C
SMILES字符串
C=CC=C
InChI
1S/C4H6/c1-3-4-2/h3-4H,1-2H2
InChI key
KAKZBPTYRLMSJV-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般描述
应用
It may be used in the synthesis of the following:
- 1-Silyl-substituted 1,3-butadienes, by [RuHCl(CO)(PCy3)2]-catalyzed silylative coupling of terminal (E)-1,3-dienes with vinylsilanes.
- Synthetic rubber and thermoplastic resins.
- Disilylated dimers by reacting with chlorosilanes.
- Octa-2,7-dien-1-ol via palladium catalyzed-hydrodimerization.
生化/生理作用
包装
Compatible with the following:
法律信息
软管倒钩
通常也和此产品一起购买
警示用语:
Danger
危险声明
危险分类
Flam. Gas 1A - Muta. 1B - Press. Gas Liquefied gas
储存分类代码
2A - Gases
WGK
WGK 3
闪点(°F)
-104.8 °F - closed cup
闪点(°C)
-76 °C - closed cup
个人防护装备
Eyeshields, Faceshields, Gloves, multi-purpose combination respirator cartridge (US)
其他客户在看
商品
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持