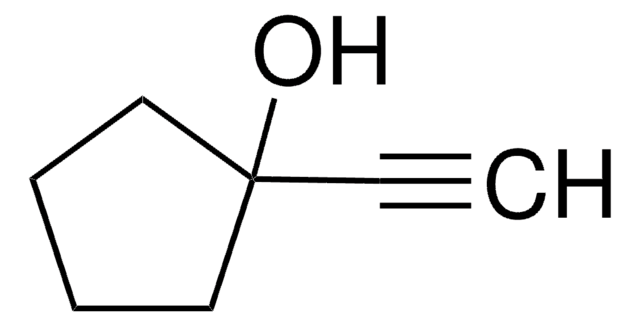
推荐产品
蒸汽密度
4.34 (vs air)
蒸汽壓力
4.5 mmHg ( 20 °C)
化驗
98%
形狀
liquid
折射率
n20/D 1.434 (lit.)
bp
150-151 °C (lit.)
密度
0.859 g/mL at 25 °C (lit.)
官能基
hydroxyl
SMILES 字串
CC(C)CC(C)(O)C#C
InChI
1S/C8H14O/c1-5-8(4,9)6-7(2)3/h1,7,9H,6H2,2-4H3
InChI 密鑰
NECRQCBKTGZNMH-UHFFFAOYSA-N
一般說明
應用
- 合成 3,5-二甲基-1-苯基-1-己烯-3-醇通过一锅钯介导的氢化氨基化/Stille 交叉 -耦合。
- 在中性离子液体(1-丁基-3-甲基咪唑四氟硼酸盐)中通过酯化反应合成 3,5-二甲基-1-己炔-3-乙酸酯。
- 通过 抗-Markovnikov 加成苯甲酸合成 3,5-二甲基-3-羟基-1-己烯-1-基苯甲酸酯。
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Irrit. 2 - Flam. Liq. 3
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
111.2 °F - closed cup
閃點(°C)
44 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
商品
Alkynes' versatility enables reactions like addition, metathesis, hydroboration, cleavage, coupling, and cycloadditions in synthetic chemistry.
Alkynes' versatility enables reactions like addition, metathesis, hydroboration, cleavage, coupling, and cycloadditions in synthetic chemistry.
Alkynes' versatility enables reactions like addition, metathesis, hydroboration, cleavage, coupling, and cycloadditions in synthetic chemistry.
Alkynes' versatility enables reactions like addition, metathesis, hydroboration, cleavage, coupling, and cycloadditions in synthetic chemistry.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门