About This Item
推荐产品
蒸汽壓力
40 mmHg ( 60 °C)
品質等級
形狀
liquid
包含
0.1% p-tert-butylcatechol as stabilizer
折射率
n20/D 1.469 (lit.)
bp
60-61 °C/40 mmHg (lit.)
密度
0.945 g/mL at 25 °C (lit.)
官能基
ester
儲存溫度
2-8°C
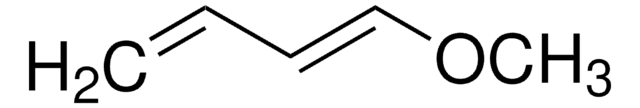
SMILES 字串
CC(=O)O\C=C\C=C
InChI
1S/C6H8O2/c1-3-4-5-8-6(2)7/h3-5H,1H2,2H3/b5-4+
InChI 密鑰
NMQQBXHZBNUXGJ-SNAWJCMRSA-N
一般說明
應用
- Diels-Alder reaction with ortho-carbazolequinones to yield benzocarbazolequinone.
- Diels-Alder reaction with diethyl ketovinylphosphonate, with and without Lewis acid assistance.
- Diels-Alder reaction with methyl acrylate to yield racemic forms of 2-hydroxy-3-cyclohexenecarboxylic acid.
It was used for the reaction with dienophiles such as maleimides, a juglone, a butyne-1,4-dione and methyl 2-(4-methylphenyl)-2H-azirine-3-carboxylate and during visible light photocatalysis. It was also used as reactant during intermolecular oxa-Pictet-Spengler cyclization.
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
91.4 °F - closed cup
閃點(°C)
33 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
商品
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门