推荐产品
品質等級
化驗
≥99%
形狀
liquid
折射率
n20/D 1.595 (lit.)
bp
181-182 °C (lit.)
mp
−5-−3 °C (lit.)
溶解度
organic solvents: miscible
water: insoluble
密度
0.996 g/mL at 25 °C (lit.)
儲存溫度
2-8°C
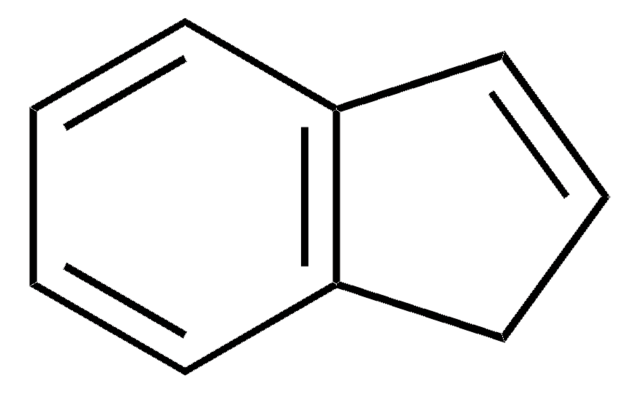
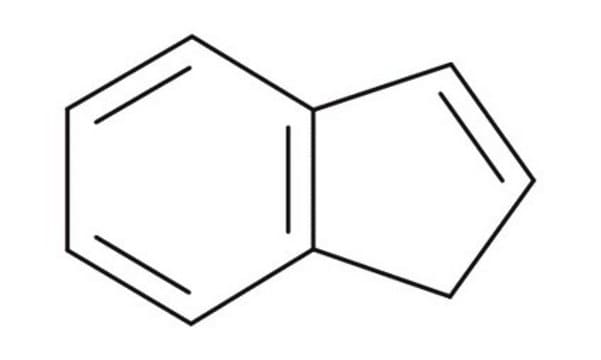
SMILES 字串
C1C=Cc2ccccc12
InChI
1S/C9H8/c1-2-5-9-7-3-6-8(9)4-1/h1-6H,7H2
InChI 密鑰
YBYIRNPNPLQARY-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
恶臭假单胞菌和红球菌将茚氧化为顺式和反式茚满醇和相关代谢物的混合物。
應用
茚被用于合成新的 C60 衍生物,茚-C60 双加合物。通过在 CH2Cl2 中的异丙基甲烷/TiCl4引发的受控阳离子聚合,将其用于制备聚茚。
訊號詞
Danger
危險聲明
危險分類
Asp. Tox. 1 - Flam. Liq. 3
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
136.4 °F - closed cup
閃點(°C)
58 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
B C Buckland et al.
Metabolic engineering, 1(1), 63-74 (2000-08-10)
Indene is oxidized to mixtures of cis- and trans-indandiols and related metabolites by Pseudomonas putida and Rhodococcus sp. isolates. Indene metabolism is consistent with monooxygenase and dioxygenase activity. P. putida resolves enantiomeric mixtures of cis-1,2-indandiol by further selective oxidation of
High glass transition temperature polyolefins obtained by the catalytic hydrogenation of polyindene.
Hahn SF and Hillmyer MA.
Macromolecules, 36(1), 71-76 (2003)
Lucas J Gursky et al.
Applied microbiology and biotechnology, 85(4), 995-1004 (2009-07-02)
The styAB genes from Pseudomonas putida CA-3, which encode styrene monooxygenase, were subjected to three rounds of in vitro evolution using error-prone polymerase chain reaction with a view to improving the rate of styrene oxide and indene oxide formation. Improvements
Regioselective synthesis of indenols by rhodium-catalyzed C-H activation and carbocyclization of aryl ketones and alkynes.
Krishnamoorthy Muralirajan et al.
Angewandte Chemie (International ed. in English), 50(18), 4169-4172 (2011-03-31)
Adam C Glass et al.
Organic letters, 10(21), 4855-4857 (2008-10-07)
A new methodology for the preparation of substituted naphthalenes starting from readily available indenones, organometal reagents, and trimethylsilyldiazomethane via a catalytic rearrangement process is described. Hindered biaryl naphthalenes, including triortho-substituted biaryls, can be accessed through our method. Our results are
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门