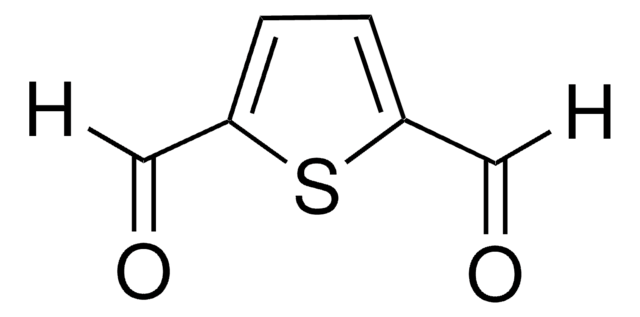
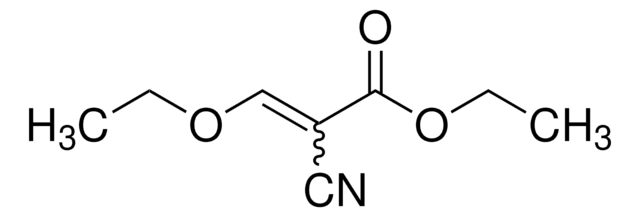
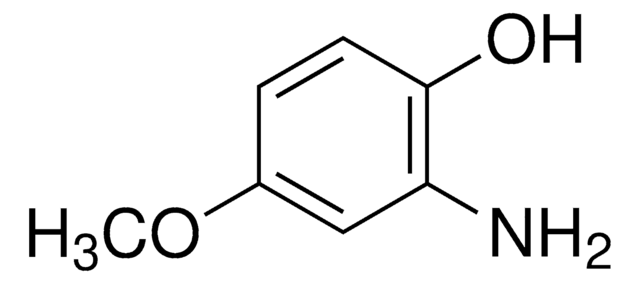
推荐产品
化驗
98%
mp
160-163 °C (lit.)
SMILES 字串
CC(C)(C)c1ccc(O)c(N)c1
InChI
1S/C10H15NO/c1-10(2,3)7-4-5-9(12)8(11)6-7/h4-6,12H,11H2,1-3H3
InChI 密鑰
RPJUVNYXHUCRMG-UHFFFAOYSA-N
應用
2-Amino-4-tert-butylphenol can be used as a reactant to prepare:
- 4-tert-butyl-2-[(pyridylmethylene)amino]phenol intermediates, which are used to synthesize biologically important 2-(pyridyl)benzoxazole derivatives.
- Prolinamide phenols, as efficient hydrophobic organocatalysts for direct asymmetric aldol reaction aldehydes and ketones in water.
- N-(2-hydroxy-4-tert-butylphenyl)-acetamide, a key intermediate to prepare uranylsalophene derivatives which can be used as selective receptors in anion sensitive membrane sensors.
- Poly(2-amino-4-tert-butylphenol) [poly(2A-4TBP)] by electrochemical or chemical oxidative polymerization reaction.
訊號詞
Warning
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Synthesis of 2-(2-, 3-, and 4-pyridyl) benzoxazoles by the reaction of phenolic Schiff bases with thianthrene cation radical.
Park MS, et al.
Journal of Heterocyclic Chemistry, 39(6), 1279-1282 (2002)
The chemical and electrochemical oxidative polymerization of 2-amino-4-tert-butylphenol
Abidi M, et al.
Electrochimica Acta, 212, 958-965 (2016)
Neutral anion receptors: synthesis and evaluation as sensing molecules in chemically modified field effect transistors.
Antonisse MMG, et al.
The Journal of Organic Chemistry, 62(26), 9034-9038 (1997)
Rationally designed 4-phenoxy substituted prolinamide phenols organocatalyst for the direct aldol reaction in water.
Zhang S-P, et al.
Tetrahedron Letters, 50(11), 1173-1176 (2009)
Rationally designed 4-phenoxy substituted prolinamide phenols organocatalyst for the direct aldol reaction in water
Z Shu-peng, et al.
Tetrahedron Letters, 50(11), 1173-1176 (2009)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门

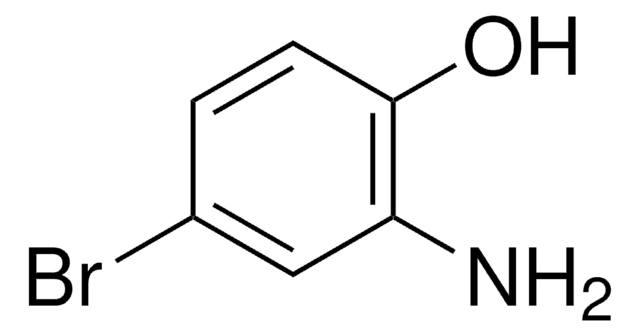
![4-(DIMETHYLAMINO)BENZALDEHYDE [4-(DIMETHYLAMINO)BENZYLIDENE]HYDRAZONE AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/268/291/5232c253-7dd7-435c-b094-6d334239d9fb/640/5232c253-7dd7-435c-b094-6d334239d9fb.png)