所有图片(1)
About This Item
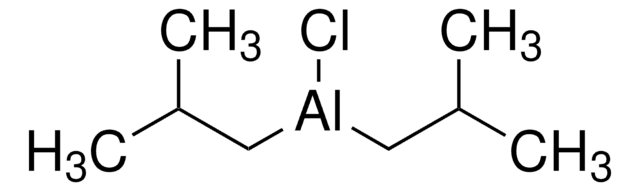
线性分子式:
[(CH3)2CHCH2]2AlH
CAS号:
分子量:
142.22
Beilstein:
4123663
MDL號碼:
分類程式碼代碼:
12352001
PubChem物質ID:
NACRES:
NA.22
推荐产品
形狀
liquid
品質等級
反應適用性
reagent type: reductant
濃度
25 wt. % in toluene
密度
0.846 g/mL at 25 °C
SMILES 字串
CC(C)C[AlH]CC(C)C
InChI
1S/2C4H9.Al.H/c2*1-4(2)3;;/h2*4H,1H2,2-3H3;;
InChI 密鑰
AZWXAPCAJCYGIA-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
二异丁基氢化铝溶液是一种强还原剂,用于将芳基丙炔酸酯转化为炔丙基醇。
二异丁基氢化铝还原剂常用于将酯还原为醛。
二异丁基氢化铝还原剂常用于将酯还原为醛。
應用
二异丁基氢化铝溶液(25 wt.% 甲苯溶液)已用于酰亚胺的还原:
- N
- -( 顺式 -2-乙烯基环己基)琥珀酰亚胺生成 1-( 顺式 -2-乙烯基环己基)-5-羟基-2-吡咯烷酮
- N -(丁-4-烯-1-基)-2 ( E )-(卡贝亚氧基亚甲基)-5-氧代-吡咯烷生成 N -(丁-4-烯-1-基)-2(E )-(甲氧基亚乙基)-5-羟基吡咯烷
- N -(庚烷-1-烯-4-基)-2 ( E )-(甲氧基亚乙基)-5-氧代-吡咯烷至 N -(庚烷-1-烯-4-基)-2 ( E )-(甲氧基亚乙基)-5-羟基吡咯烷
- N -( 顺 -乙烯基环己基)-2-(甲氧基亚乙基)-5-氧代-吡咯烷至相关-(3a R , SS ,5a S ,Sa R )-1 ( E )-(碳乙氧基亚甲基)-5-(甲酰氧基)十二氢吡咯并 [1,2- a 喹啉
用于Pd催化的仲烷基溴的还原脱溴过程。过苄基化呋喃糖苷的O-脱苄基和开环。方便从 ZrCp2Cl2 和DIBAL-H原位生成 HZrCp2Cl。
法律資訊
Sure/Seal is a trademark of Sigma-Aldrich Co. LLC
訊號詞
Danger
危險分類
Aquatic Chronic 3 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Pyr. Liq. 1 - Repr. 2 - Skin Corr. 1B - STOT RE 2 Inhalation - STOT SE 3 - Water-react 1
標靶器官
Central nervous system
安全危害
儲存類別代碼
4.2 - Pyrophoric and self-heating hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
39.2 °F - closed cup
閃點(°C)
4 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
其他客户在看
Preparation of arylpropiolate esters from trichlorocyclopropenium cation and elaboration of the esters to unsymmetrical 1, 4-pentadiyn-3-ones and unsymmetrical tellurapyranones
Wadsworth, et al.
The Journal of Organic Chemistry, 52, 3662-3668 (1987)
Vinylogous N-acyliminium ion cyclizations: application to the synthesis of depentylperhydrogephyrotoxin.
Hart DJ
The Journal of Organic Chemistry, 46(2), 367-373 (1981)
Chemoselective Reduction of Esters to Aldehydes by Potassium Diisobutyl-t-butoxyaluminum Hydride (PDBBA).
Chae MJ, et al.
Bull. Korean Chem. Soc., 28(12), 2517-2517 (2007)
D J Kopecky et al.
The Journal of organic chemistry, 65(1), 191-198 (2000-05-18)
An optimized protocol for the DIBALH reductive acetylation of acyclic esters and diesters is described. This reductive acetylation procedure allows a wide variety of esters to be converted into the corresponding alpha-acetoxy ethers in good to excellent yields. It was
J Marco-Contelles et al.
Carbohydrate research, 335(1), 63-70 (2001-09-13)
The reaction of DIBALH with bis(heteroannulated)-pyranosides containing the perhydrofuro[2,3-b]pyran moiety is described. The hydride attack at the anomeric carbon (C-9a) resulted in the exclusive tetrahydrofuran ring opening. The selectivity of this reaction has been evaluated as other benzylidene acetals built
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门