推荐产品
化驗
98%
形狀
liquid
折射率
n20/D 1.507 (lit.)
bp
125-130 °C (lit.)
密度
1.45 g/mL at 25 °C (lit.)
官能基
chloro
儲存溫度
2-8°C
SMILES 字串
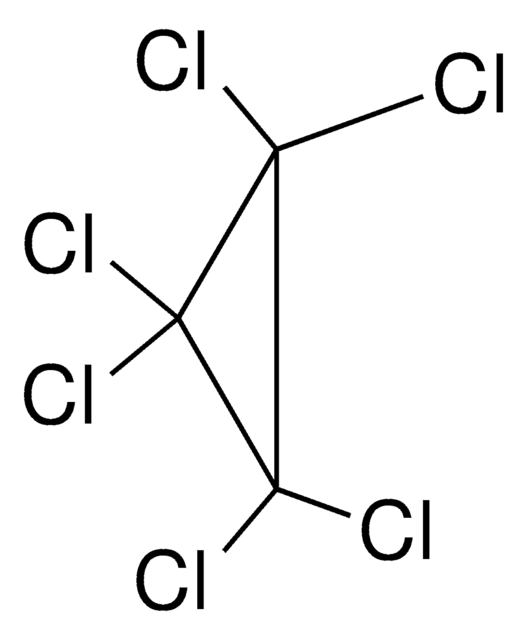
ClC1=C(Cl)C1(Cl)Cl
InChI
1S/C3Cl4/c4-1-2(5)3(1,6)7
InChI 密鑰
BLZOHTXDDOAASQ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
四氯环丙烯经过电化学还原 叔 丁基二甲基硅烷化反应生成 1,2,3- 三 ( 叔 丁基二甲基硅基)环丙烯 。它在二氯甲烷和 AlCl 3 中与二茂铁发生烷基化反应生成 2,3-二茂铁环丙烯酮 。
應用
采用四氯环丙烯,制备用于合成 3,3-二乙基和 3,3-二苄基-1,2-二茂铁基环丙烯的起始试剂 。
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
从最新的版本中选择一种:
分析证书(COA)
Lot/Batch Number
其他客户在看
H A Buchholz et al.
Proceedings of the National Academy of Sciences of the United States of America, 96(18), 10003-10005 (1999-09-01)
Electrochemical reductive tert-butyldimethylsilylation of tetrachlorocyclopropene to 1,2,3-tris(tert-butyldimethylsilyl)cyclopropene, a potential strained precursor for Diels-Alder and related cycloaddition reactions, is described. By hydride abstraction with nitrosonium tetrafluoroborate, 1,2,3-tris(tert-butyldimethylsilyl)cyclopropene is ionized quantitatively to Hückeloid 2pi aromatic tris(tert-butyldimethylsilyl)cyclopropenium tetrafluoroborate.
Noam Y Steinman et al.
Pharmaceutics, 12(8) (2020-08-23)
Non-viral vectors for the transfection of genetic material are at the frontier of medical science. In this article, we introduce for the first time, cyclopropenium-containing nanoparticles as a cationic carrier for gene transfection, as an alternative to the common quaternary
Elena I Klimova et al.
Organic & biomolecular chemistry, 1(24), 4458-4464 (2004-01-20)
Reactions of 2,3-diferrocenylcyclopropenone 1 with ethyl- and benzylmagnesium chlorides afford 3,3-diethyl-and 3,3-dibenzyl- 1,2-diferrocenylcyclopropenes 2 and 3, respectively, and products of nucleophilic opening of the three-membered ring resulting from the addition of RMgCl to the carbonyl group, viz., saturated ketones(4,5-diferrocenylheptan-3-ones 4a,b
Jesús García-Valdés et al.
Journal of inorganic biochemistry, 197, 110689-110689 (2019-05-18)
Bis-cations with two 2,3-diferrocenylcyclopropenium fragments 3a-d, and the cis-2-(1,2-diferrocenylvinyl)-2-imidazolinium tetrafluoroborates 4a, d or the cis-2-(1,2-diferrocenylvinyl)-3,4,5,6-tetrahydropyrimidin-2-ium tetrafluoroborates 4b, c were obtained by interactions of 2,3-diferrocenyl-1-ethoxycyclopropenium tetrafluoroborate 1 with bis-1,4-N,N-(2a, d) or bis-1,5-N,N-(2b, c) nucleophiles. The reactions of 3a-d with sodium azide
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门