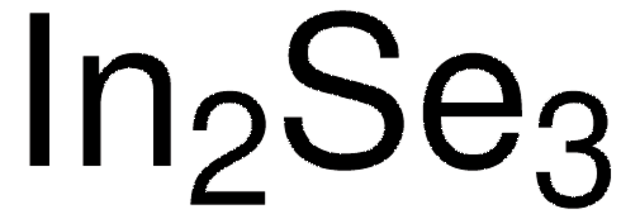400947
Manganese(II) sulfide
~100 mesh particle size
Synonym(s):
Manganese sulfide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
MnS
CAS Number:
Molecular Weight:
87.00
EC Number:
MDL number:
UNSPSC Code:
26111700
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
form
powder
Quality Level
particle size
~100 mesh
SMILES string
S=[Mn]
InChI
1S/Mn.S
InChI key
CADICXFYUNYKGD-UHFFFAOYSA-N
Related Categories
General description
MnS can exist in three polymorphs; α,β,γ, of these, the α phase is most thermodynamically stable.
Packaging
Packaged in glass bottles
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
536.0 °F
Flash Point(C)
280 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hydrothermal Synthesis of Pure r-Phase Manganese(II) Sulfide without the Use of Organic Reagents
Michel FM, et al.
Chemistry of Materials, 18, 1726-1736 (2006)
Xiang V Zhang et al.
Journal of the American Chemical Society, 126(36), 11247-11253 (2004-09-10)
Photoelectrochemistry on mineral surfaces has the potential to play a central role in the prebiotic syntheses of building blocks for biomolecules. In this study, photoreduction of C(+IV) as bicarbonate is used as a probe to investigate the photoelectrochemical properties of
Lei Zhang et al.
Angewandte Chemie (International ed. in English), 51(29), 7267-7270 (2012-06-12)
Kept cubic: MnS microboxes, which act as an anode material for lithium ion batteries, are synthesized by a simple H(2)S gas sulfidation approach (TEM images show porous and hollow microcubes and a microbox). The formation of the single crystals is
Keita Ikeue et al.
ChemSusChem, 4(2), 269-273 (2010-09-30)
The effect of metal doping (i.e., with Cr, Fe, Ni, Cu, Zn, Ag and Sn) on the crystal structure of hydrothermally synthesized Mn(1-x)Cd(x) S (where x≈0.1) is studied with the aim of enhancing photocatalytic activity. In contrast to the low-crystalline
Taisen Zuo et al.
Journal of the American Chemical Society, 132(19), 6618-6619 (2010-04-30)
The red photoluminescence of Mn dopants in MnS-CdS heteronanostructures has been observed for the first time. The red photoluminescence at 650 nm derives from emission due to the (4)T(1) --> (6)A(1) transition of Mn(2+) dopants in a CdS matrix exposed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service