SML0320
FR180204
≥98% (HPLC)
Synonyme(s) :
5-(2-Phenyl-pyrazolo[1,5-a]pyridin-3-yl)-1H-pyrazolo[3,4-c]pyridazin-3-ylamine, 5-(2-Phenylpyrazolo[1,5-a]pyridin-3-yl)-1H-pyrazolo[3,4-c]pyridazin-3-amine, FR 180204
About This Item
Produits recommandés
Niveau de qualité
Pureté
≥98% (HPLC)
Forme
powder
Couleur
faintly yellow to dark yellow
Solubilité
DMSO: ≥10 mg/mL
Température de stockage
2-8°C
Chaîne SMILES
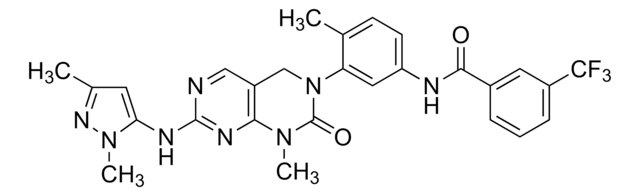
Nc1n[nH]c2nnc(cc12)-c3c(nn4ccccc34)-c5ccccc5
InChI
1S/C18H13N7/c19-17-12-10-13(20-22-18(12)23-21-17)15-14-8-4-5-9-25(14)24-16(15)11-6-2-1-3-7-11/h1-10H,(H3,19,21,22,23)
Clé InChI
XVECMUKVOMUNLE-UHFFFAOYSA-N
Application
- to block extracellular-signal-regulated kinase (ERK) in order to validate whether homeobox B7 (HOXB7) regulates the migration and proliferation process via AKT/mitogen-activated protein kinases (MAPK) signaling
- of extracellular-signal-regulated kinase (ERK) to study the effects of signaling pathway inhibitors on differentiation and cell traction stress
- to determine if phosphorylation of this signaling protein is essential for mesenchymal stromal cell (MSC) derived therapeutic efficacy
Actions biochimiques/physiologiques
Caractéristiques et avantages
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique