S8195
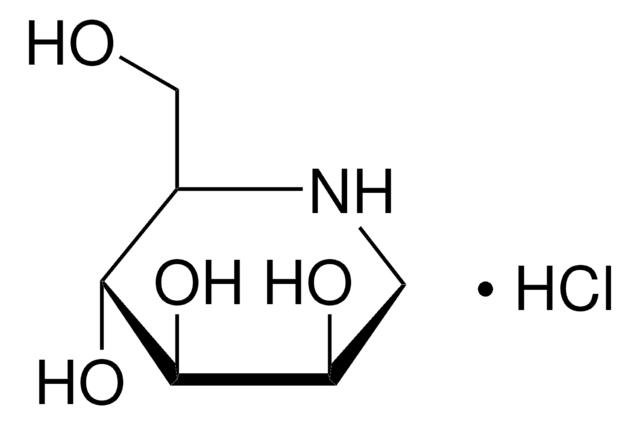
Swainsonine
from Metarrhizium anisopliae, ≥98% (TLC)
Synonyme(s) :
(1S,2R,8R,8aR)-1,2,8-Octahydroindolizidinetriol
About This Item
Produits recommandés
Source biologique
Metarrhizium anisopliae
Niveau de qualité
Essai
≥98% (TLC)
Forme
lyophilized powder
Conditions de stockage
(Keep container tightly closed in a dry and well-ventilated place.)
Couleur
white to faint yellow
Solubilité
H2O: soluble 1 mg/mL
Spectre d'activité de l'antibiotique
neoplastics
Mode d’action
enzyme | inhibits
Température de stockage
2-8°C
Chaîne SMILES
O[C@@H]1CCCN2C[C@@H](O)[C@@H](O)C12
InChI
1S/C8H15NO3/c10-5-2-1-3-9-4-6(11)8(12)7(5)9/h5-8,10-12H,1-4H2/t5-,6-,7?,8-/m1/s1
Clé InChI
FXUAIOOAOAVCGD-DCDLSZRSSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Application
Actions biochimiques/physiologiques
Conditionnement
Notes préparatoires
Autres remarques
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique