A1729
Activin B human
≥90% (SDS-PAGE), recombinant, expressed in CHO cells, lyophilized, suitable for cell culture
About This Item
Produits recommandés
product name
Activin B human, recombinant, expressed in CHO cells, suitable for cell culture
Source biologique
human
Niveau de qualité
Produit recombinant
expressed in CHO cells
Pureté
≥90% (SDS-PAGE)
Forme
lyophilized
Puissance
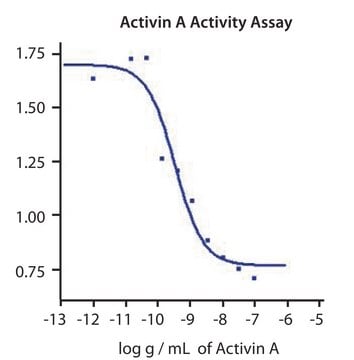
0.30-1.50 ng/mL ED50
Poids mol.
calculated mol wt ~14.5 kDa
Conditionnement
pkg of 5 μg
Conditions de stockage
avoid repeated freeze/thaw cycles
Technique(s)
cell culture | mammalian: suitable
Impuretés
endotoxin, tested
Température de stockage
−20°C
Actions biochimiques/physiologiques
Similar to activin-A, activin-B modulates follicle stimulating hormone (FSH) secretion and hemoglobin synthesis, DePaolo LV, et al. (1992); Mason AJ, et al. (1989). It is involved in regulation of the menstrual cycle, Liu J, et al. (2001).
Activin-B is not essential for embryo development or survival, but it does have important roles in development. It stimulates spermatogonial proliferation, Mather JP, et al. (1990) and is localized in specific cells, gonocytes and interstitial Leydig cells, and tissues, rete testis and epididymal epithelium, associated with human testis duct system development, Anderson RA, et al. (2002). Activin-B is found in follicle cells surrounding oocytes, Dohrmann CE, et al. (1993) and been shown to increase the rate of oocytes maturation in a dose and time dependent manner, Pang Y and Ge W. (1999, 2002). It is required for successful mammogenesis leading to lactation, Robinson GW and Hennighausen L. (1997). Recent studies suggest that activin-B may regulate adipocyte differentiation, Kogame, M, et al. (2006).
Activin-B has been linked to several aspects of embryo development. Activin-B is first detected in blastula-stage embryos, Thomsen G, et al. (1990) after midblastula transition and homogeneously distributed during blastula and early gastrula stages. It becomes restricted to the dorso-anterior region in neurula-stage embryos and by early tailbud stage it is restricted to brain, eye anlagen, visceral pouches, otic vesicles and the anterior notochord, Dohrmann CE, et al. (1993). Activin-A and -B localizations are different in 3.5 and 4.5 day mouse blastocyst, Paulusman CC, et al. (1994).
Nakamura, T et al. (1992) suggested that the primary function of Activin-B may involve mesoderm-inducing activity and early development modulation. Schrewe H, et al (1994) and Vassalli A, et al. (1994), reported that activin-B was not essential for survival or for mesoderm formation (in mouse), but that it played a role in late fetal development and female fecundity. A role for activin-B in axial formation was suspected because ectopic expression produced second body axis embryos, Thomsen G, et al. (1990), Mitrani E, et al. (1990).
The roles of activin-B during embryo development are starting to emerge. Activin-B may modulate gap junction permeability in embryos, Olson DJ and Moon RT. (1992). Activin-B has been shown to modulate the morphogenesis of the roof plate (RP) of midbrain wherein it inhibits roof plate differentiation, Alexandre P, et al. (2006). Activin-B signals cell cycle arrest in cells of the involuting dorsal axial mesoderm, Ramis JM, et al. (2007).
Activin-B is involved in the development of the adrenal gland and pancreas. It is present in normal adrenal medulla, but absent in the cortex, Salmenkivi K et al. (2001). Interestingly, Activin-B may be a marker for benign adrenal pheochromocytomas, Salmenkivi K et al. (2001).
The role of activin-B in pancreas development is of particular interest because of efforts to use human embryonic stem cells (hESC) as precursors to make insulin producing cells for treatment of diabetes. Activin-B has been shown to promote expression of the pancreas marker Pdx1 gene in cells of differentiated embryoid bodies (EB), in culture, Frandsen U, et al. (2007). Most recently, Jafary H, et al. (2008) induced insulin-secreting cells for ES by adding activin-B to nestin-positive selection protocol cell.
Forme physique
Remarque sur l'analyse
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Inhalation - Eye Irrit. 2
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique