147753
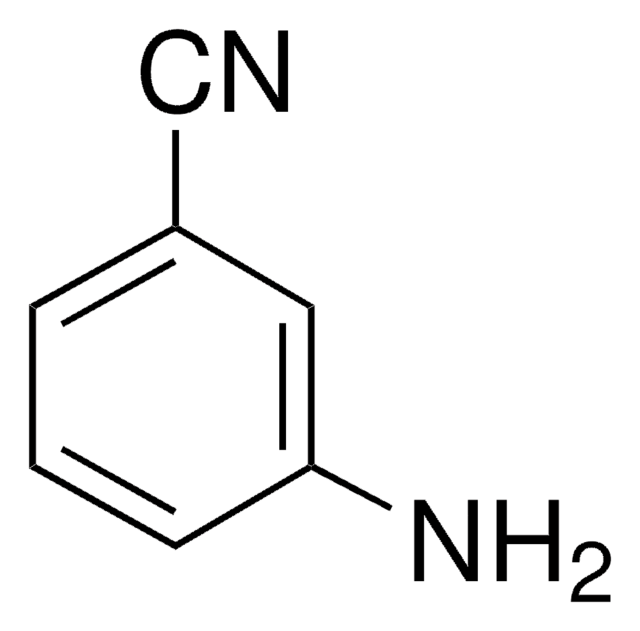
4-Aminobenzonitrile
98%
Synonym(s):
4-Cyanoaniline
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2NC6H4CN
CAS Number:
Molecular Weight:
118.14
Beilstein:
774507
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
83-85 °C (lit.)
SMILES string
Nc1ccc(cc1)C#N
InChI
1S/C7H6N2/c8-5-6-1-3-7(9)4-2-6/h1-4H,9H2
InChI key
YBAZINRZQSAIAY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-Aminobenzonitrile was used as derivatization reagent in capillary zone electrophoretic analysis of aldoses, ketoses and uronic acid. It was used in the synthesis of methacrylic monomers containing pendant azobenzene structures and polythiophenes containing an azobenzene moiety in the side-chain.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Muta. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ranjit Kulkarni et al.
Nature communications, 10(1), 3228-3228 (2019-07-22)
Fully-aromatic, two-dimensional covalent organic frameworks (2D COFs) are hailed as candidates for electronic and optical devices, yet to-date few applications emerged that make genuine use of their rational, predictive design principles and permanent pore structure. Here, we present a 2D
Synthesis and characterization of novel azobenzene methacrylate monomers.
Nicolescu FA, et al.
Designed Monomers and Polymers, 12(6), 553-563 (2009)
Synthesis of azobenzene-containing polythiophenes and photoinduced anisotropy.
Aubertin F and Zhao Y.
Journal of Polymer Science: Part A, General Papers, 42(14), 3445-3455 (2004)
Isabella Rustighi et al.
Electrophoresis, 30(15), 2632-2639 (2009-07-22)
N-linked or O-linked glycans derived from glycoprotein processing carry, an N-acetylglucosamine or an N-acetylgalactosamine respectively, at their reducing termini. The presence of the N-acetylamino group on C-2 of reducing sugar residues has been reported to hamper the derivatization reaction with
[Benzonitrile and its derivatives: action of para-aminobenzonitrile on the cardiovascular system].
S L Cheav et al.
Annales pharmaceutiques francaises, 44(5), 363-372 (1986-01-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service