Wichtige Dokumente
N6287
Nutlin-3
≥98% (HPLC), powder, Mdm2 antagonist
Synonym(e):
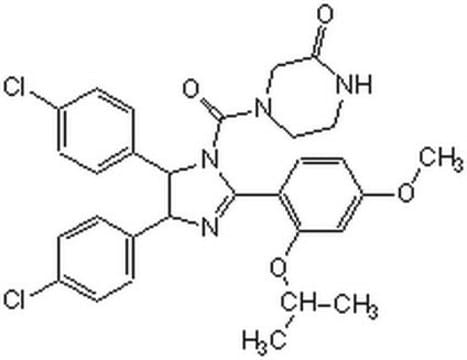
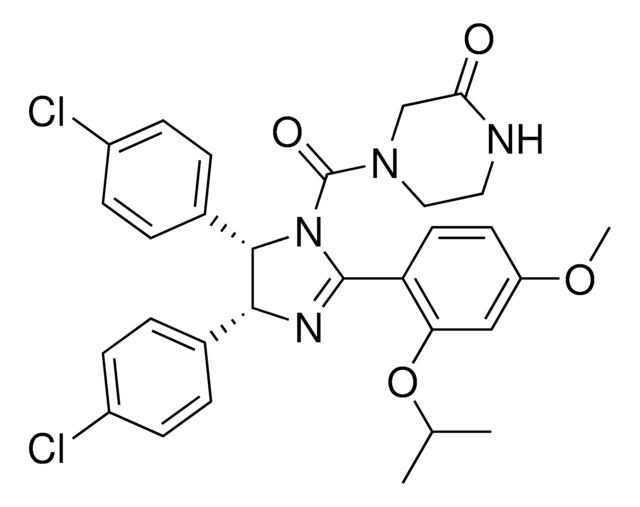
(±)-4-[4,5-Bis(4-chlorophenyl)-2-(2-isopropoxy-4-methoxy-phenyl)-4,5-dihydro-imidazole-1-carbonyl]-piperazin-2-one
About This Item
Empfohlene Produkte
Produktbezeichnung
Nutlin-3, ≥98% (HPLC), powder
ligand
nutlin-3
Qualitätsniveau
Assay
≥98% (HPLC)
Form
powder
Eignung der Reaktion
reagent type: ligand
Löslichkeit
DMSO: 20 mg/mL
H2O: insoluble
Ersteller
Roche
Versandbedingung
wet ice
Lagertemp.
−20°C
SMILES String
O=C(N1CC(NCC1)=O)N2C(C3=CC=C(Cl)C=C3)C(C4=CC=C(Cl)C=C4)N=C2C5=C(OC(C)C)C=C(OC)C=C5
InChI
1S/C30H30Cl2N4O4/c1-18(2)40-25-16-23(39-3)12-13-24(25)29-34-27(19-4-8-21(31)9-5-19)28(20-6-10-22(32)11-7-20)36(29)30(38)35-15-14-33-26(37)17-35/h4-13,16,18,27-28H,14-15,17H2,1-3H3,(H,33,37)/t27-,28+/m1/s1
InChIKey
BDUHCSBCVGXTJM-IZLXSDGUSA-N
Anwendung
- as a drug to stimulate p53 functions in gene transfer experiment
- to inject worms to verify the prevalent role of translationally controlled tumor protein (TCTP) in posterior amputated E. eugeniae
- as a p53 activator in cyclotherapy studies
- as an mdm2 inhibitor to know its effect on p53 levels, cleaved caspase 3 and Poly (ADP-ribose) polymerase (PARP) cleavage
Biochem./physiol. Wirkung
Leistungsmerkmale und Vorteile
Sonstige Hinweise
Hinweis zur Analyse
Rechtliche Hinweise
Ähnliches Produkt
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Die passende Version wird nicht angezeigt?
Wenn Sie eine bestimmte Version benötigen, können Sie anhand der Lot- oder Chargennummer nach einem spezifischen Zertifikat suchen.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.