Wichtige Dokumente
31571
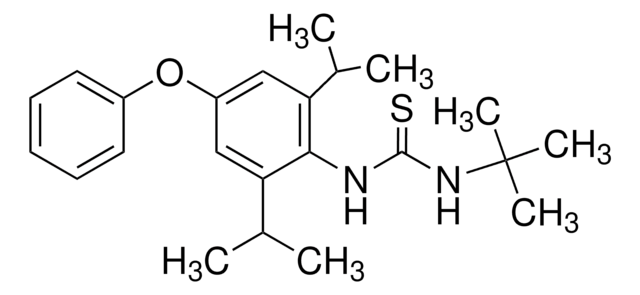
Diafenthiuron
PESTANAL®, analytical standard
Synonym(e):
1-tert-Butyl-3-(2,6-diisopropyl-4-phenoxyphenyl)-thiourea, N-[2,6-Bis(1-methylethyl)-4-phenoxyphenyl]-N′-(1,1-dimethylethyl)-thiourea
About This Item
Empfohlene Produkte
Qualität
analytical standard
Qualitätsniveau
Produktlinie
PESTANAL®
Haltbarkeit
limited shelf life, expiry date on the label
Methode(n)
HPLC: suitable
gas chromatography (GC): suitable
Anwendung(en)
agriculture
environmental
Format
neat
SMILES String
CC(C)c1cc(Oc2ccccc2)cc(C(C)C)c1NC(=S)NC(C)(C)C
InChI
1S/C23H32N2OS/c1-15(2)19-13-18(26-17-11-9-8-10-12-17)14-20(16(3)4)21(19)24-22(27)25-23(5,6)7/h8-16H,1-7H3,(H2,24,25,27)
InChIKey
WOWBFOBYOAGEEA-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Anwendung
- Cotton and groundnut oil by quick, easy, cheap, effective, rugged, and safe (QuEChERS) extraction procedure, low-temperature freezing and dispersive clean-up followed by quantification using gas chromatography (GC) equipped with electron capture detector (ECD) as well as flame photometric detector (FPD) and liquid chromatography-tandem mass spectrometry (LC-MS/MS).
- Tomatoes by QuEChERS extraction and LC combined with triple quadrupole MS/MS with electrospray ionization source (ESI).
- Fruit juice samples by ionic liquid-assisted liquid-phase microextraction based on the solidification of floating organic droplets (ILSFOD-LLME) and high performance liquid chromatography (HPLC) equipped with a variable-wavelength detector (VWD).
- Water and wastewater by solid phase extraction (SPE) and LC combined with time-of-flight (TOF) MS.
Empfohlene Produkte
Rechtliche Hinweise
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 3 Inhalation - Aquatic Acute 1 - Aquatic Chronic 1 - STOT RE 2
Lagerklassenschlüssel
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flammpunkt (°F)
>300.2 °F - closed cup
Flammpunkt (°C)
> 149 °C - closed cup
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.