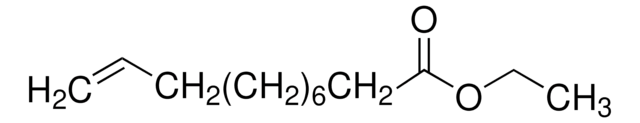W304409
L-(+)-Tartarsäure
≥99.7%, FCC, FG
Synonym(e):
(2R,3R)-(+)-Weinsäure, L-Threarsäure
About This Item
Empfohlene Produkte
Biologische Quelle
synthetic
Qualitätsniveau
Qualität
FG
Halal
Agentur
meets purity specifications of JECFA
Einhaltung gesetzlicher Vorschriften
EU Regulation 1334/2008 & 178/2002
FCC
FDA 21 CFR 163.110
FDA 21 CFR 163.111
FDA 21 CFR 163.112
FDA 21 CFR 184.1099
Dampfdichte
5.18 (vs air)
Assay
≥99.7%
Form
crystalline powder
Optische Aktivität
[α]20/D +12.5°, c = 20 in H2O
Selbstzündungstemp.
797 °F
mp (Schmelzpunkt)
170-172 °C (lit.)
Löslichkeit
water: soluble 150 g/L at 25 °C
Kationenspuren
As: ≤3 ppm
Cd: ≤1 ppm
Hg: ≤1 ppm
heavy metals (as Pb): ≤2 ppm
Anwendung(en)
flavors and fragrances
Dokumentation
see Safety & Documentation for available documents
Nahrungsmittelallergen
no known allergens
Organoleptisch
odorless
SMILES String
O[C@H]([C@@H](O)C(O)=O)C(O)=O
InChI
1S/C4H6O6/c5-1(3(7)8)2(6)4(9)10/h1-2,5-6H,(H,7,8)(H,9,10)/t1-,2-/m1/s1
InChIKey
FEWJPZIEWOKRBE-JCYAYHJZSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
- Terahertz-spectroscopy for non-destructive determination of crystallinity of L-tartaric acid in smartFilms and tablets made from paper.: This study leverages terahertz spectroscopy to assess the crystallinity of L-(+)-tartaric acid in innovative pharmaceutical applications, enhancing non-destructive testing methods for quality control (Ornik et al., 2020 May). Link to the article.
- Enhanced pulmonary absorption of poorly soluble itraconazole by micronized cocrystal dry powder formulations.: Research shows the use of L-(+)-tartaric acid in cocrystal formulations with itraconazole to improve its pulmonary absorption, demonstrating a significant advancement in drug delivery technologies (Karashima et al., 2017 Jun). Link to the article.
- Physicochemical Evaluation and Developability Assessment of Co-amorphouses of Low Soluble Drugs and Comparison to the Co-crystals.: This article discusses the role of L-(+)-tartaric acid in enhancing the solubility and bioavailability of pharmaceuticals through co-amorphous systems, offering a critical insight into drug formulation strategies (Yamamoto et al., 2016 Dec). Link to the article.
- Functionalized polycarbonate derived from tartaric acid: enzymatic ring-opening polymerization of a seven-membered cyclic carbonate.: This research explores the synthesis of biodegradable polymers from L-(+)-tartaric acid, emphasizing its utility in developing environmentally friendly materials (Wu et al., 2008 Oct). Link to the article.
Signalwort
Danger
H-Sätze
P-Sätze
Gefahreneinstufungen
Eye Dam. 1
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 1
Flammpunkt (°F)
302.0 °F - closed cup
Flammpunkt (°C)
150 °C - closed cup
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.