Wichtige Dokumente
W281107
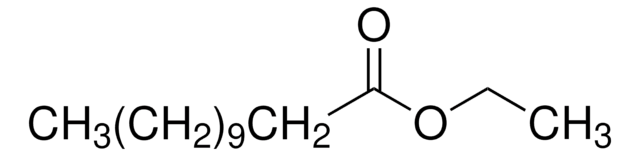
Octyl octanoate
≥98%, FG
Synonym(e):
Octyl caprylate
About This Item
Empfohlene Produkte
Biologische Quelle
synthetic
Qualitätsniveau
Qualität
FG
Kosher
Agentur
meets purity specifications of JECFA
Einhaltung gesetzlicher Vorschriften
EU Regulation 1334/2008 & 872/2012
Assay
≥98%
Brechungsindex
n20/D 1.435 (lit.)
bp
307 °C (lit.)
mp (Schmelzpunkt)
−18 °C (lit.)
Dichte
0.859 g/mL at 25 °C (lit.)
Anwendung(en)
flavors and fragrances
Dokumentation
see Safety & Documentation for available documents
Nahrungsmittelallergen
no known allergens
Organoleptisch
coconut; oily; fruity; sweet
SMILES String
CCCCCCCCOC(=O)CCCCCCC
InChI
1S/C16H32O2/c1-3-5-7-9-11-13-15-18-16(17)14-12-10-8-6-4-2/h3-15H2,1-2H3
InChIKey
DJNTZVRUYMHBTD-UHFFFAOYSA-N
Verwandte Kategorien
Lagerklassenschlüssel
10 - Combustible liquids
WGK
nwg
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.