Alle Fotos(2)
Wichtige Dokumente
T90301
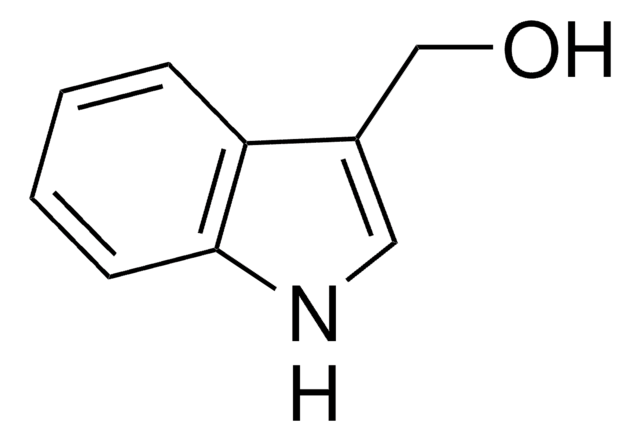
3-(2-Hydroxyethyl)-indol
97%
Synonym(e):
3-Indolethanol, ‘Tryptophol’
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Empirische Formel (Hill-System):
C10H11NO
CAS-Nummer:
Molekulargewicht:
161.20
Beilstein:
125553
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
97%
mp (Schmelzpunkt)
56-59 °C (lit.)
SMILES String
OCCc1c[nH]c2ccccc12
InChI
1S/C10H11NO/c12-6-5-8-7-11-10-4-2-1-3-9(8)10/h1-4,7,11-12H,5-6H2
InChIKey
MBBOMCVGYCRMEA-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
Reactant for preparation of:
- Inhibitors of the C-terminal domain of RNA polymerase II and their antitumor activities
- Anti-HIV-1 agents
- Inhibitors of Protein-Protein Interactions
- Partial agonists of the serotonin 5-HT1A receptor
- Growth hormone secretagogues
- Vascular endothelial growth factor (VEGF) inhibitors
- A2B adenosine receptor ligands
- Potential detoxification inhibitors of the crucifer phytoalexin brassinin
- Inhibitors of interleukine 6
- Dual binding site acetylcholinesterase inhibitors
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Chuan Liu et al.
Organic letters, 14(17), 4525-4527 (2012-08-16)
Copper(I)-catalyzed dearomative arylation and vinylation of 2-substituted tryptophols were realized with a subsequent cyclization reaction. The cascade dearomatization sequence provided versatile furoindoline derivatives with two quaternary carbon centers in good to excellent yields.
Ivan Kosalec et al.
Arhiv za higijenu rada i toksikologiju, 62(1), 41-49 (2011-03-23)
Tryptophol is an aromatic alcohol and secondary metabolite of the opportunistic fungus Candida albicans. Although its toxicity profile at cell level has been poorly investigated, recent data point to cytotoxic, cytostatic, and genotoxic effects in lymphocytes and the induction of
Olivier Vandeputte et al.
Applied and environmental microbiology, 71(3), 1169-1177 (2005-03-05)
The role and metabolism of indole-3-acetic acid in gram-negative bacteria is well documented, but little is known about indole-3-acetic acid biosynthesis and regulation in gram-positive bacteria. The phytopathogen Rhodococcus fascians, a gram-positive organism, incites diverse developmental alterations, such as leafy
Changyuan Lu et al.
Journal of the American Chemical Society, 131(36), 12866-12867 (2009-09-10)
Human indoleamine 2,3-dioxygenase (hIDO) is an intracellular heme-containing enzyme, which catalyzes the initial and rate-determining step of L-tryptophan (L-Trp) metabolism via the kynurenine pathway. Due to its immunosuppressive function, hIDO has been recognized as an important drug target for cancer.
Hao Chen et al.
Genes & development, 20(9), 1150-1161 (2006-04-19)
Many fungi undergo a developmental transition from a unicellular yeast form to an invasive filamentous form in response to environmental cues. Here we describe a quorum signaling pathway that links environmental sensing to morphogenesis in Saccharomyces cerevisiae. Saccharomyces cells secrete
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.