B32602
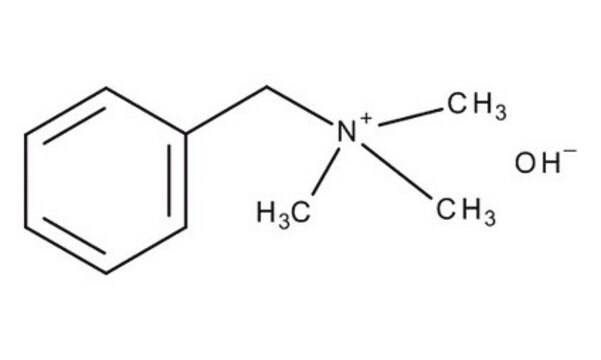
Benzyltrimethylammoniumhydroxid -Lösung
40 wt. % in methanol
Synonym(e):
N,N,N-Trimethyl-N-benzylammonium hydroxide, N,N,N-trimethyl-1-phenylmethanaminium hydroxide, N,N,N-trimethylbenzenemethanaminium hydroxide, Triton B
About This Item
Empfohlene Produkte
Form
liquid
Qualitätsniveau
Konzentration
40 wt. % in methanol
Dichte
0.92 g/mL at 25 °C
SMILES String
[OH-].C[N+](C)(C)Cc1ccccc1
InChI
1S/C10H16N.H2O/c1-11(2,3)9-10-7-5-4-6-8-10;/h4-8H,9H2,1-3H3;1H2/q+1;/p-1
InChIKey
NDKBVBUGCNGSJJ-UHFFFAOYSA-M
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
- A catalyst in the nitroaldol condensation reaction
- A structure-directing agent in the synthesis of high-silica aluminosilicate zeolite chabazite type zeolite by hydrothermal method
- A ionic liquid precursor for the fabrication of nanostructured ZnO particles
Signalwort
Danger
Gefahreneinstufungen
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1A - STOT SE 1
Zielorgane
Eyes,Central nervous system
Lagerklassenschlüssel
3 - Flammable liquids
WGK
WGK 2
Flammpunkt (°F)
51.8 °F - closed cup
Flammpunkt (°C)
11 °C - closed cup
Persönliche Schutzausrüstung
Faceshields, Gloves, Goggles
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.
![1,8-Diazabicyclo[5.4.0]undec-7-en (1,5-5) 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)






![1,4-Diazabicyclo[2.2.2]octan ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)







