914975
KB03-SLF
≥95%
Synonym(e):
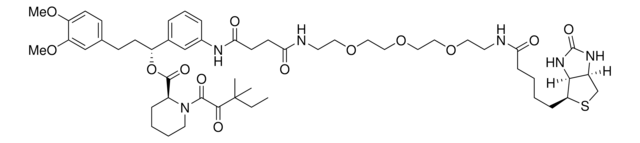
(R)-1-(3-(1-(3-(2-Chloroacetamido)-5-(trifluoromethyl)phenyl)-1,5-dioxo-9,12-dioxa-2,6-diazapentadecan-15-amido)phenyl)-3-(3,4-dimethoxyphenyl)propyl (S)-1-(3,3-dimethyl-2-oxopentanoyl)piperidine-2-carboxylate, Electrophilic PROTAC®, Heterobifunctional conjugate for E3 ubiquitin ligase discovery
About This Item
Empfohlene Produkte
ligand
electrophilic fragment
Qualitätsniveau
Assay
≥95%
Form
powder
Lagertemp.
−20°C
SMILES String
ClCC(NC1=CC(C(NCCC(NCCOCCOCCC(NC2=CC=CC([C@H](OC([C@@H]3CCCCN3C(C(C(C)(C)CC)=O)=O)=O)CCC4=CC(OC)=C(OC)C=C4)=C2)=O)=O)=O)=CC(C(F)(F)F)=C1)=O
Verwandte Kategorien
Anwendung
This proteomic approach to E3 discovery was demonstrated by Zhang et al in the discovery that DCAF16 mediated nuclear FKBP12 degradation via KB02-SLF.
Additional electrophilic PROTACs were developed incorporating scout fragments with broad cysteine reactivity:
- KB02-SLF (914738) containing chloroacetamide scout fragment 912131
- KB05-SLF (913715) containing acrylamide scout fragment 911798
Related tools:
- Additional bifunctional tools for FKPB12 variants: dTAG-13 (SML2601 for FKBP12F36V) and Biotin-SLF (914223 for FKBP12)
- Inhibitors useful in validation of proteasomal-mediated degradation: MG123 (SML1135) and MLN4924 (5.05477 for Cullin-RING ubiquitin ligases that regulate neddylation of Cullin proteins)
- Cereblon (CRBN) affinity probe: Biotin-Thalidomide (913979)
Sonstige Hinweise
Rechtliche Hinweise
Ähnliches Produkt
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Leider sind derzeit keine COAs für dieses Produkt online verfügbar.
Wenn Sie Hilfe benötigen, wenden Sie sich bitte an Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.

![(R)-2-(5-Bromo-4-(4-chlorobenzyl)-7-fluoro-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetic acid](/deepweb/assets/sigmaaldrich/product/structures/321/793/9c59bf88-c483-4a85-a559-53ba29e916d1/640/9c59bf88-c483-4a85-a559-53ba29e916d1.png)


![[3-(2-carboxyethyl)phenyl]boronic acid AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/265/067/68263dcf-5afc-49a6-982b-0394e48bf9c2/640/68263dcf-5afc-49a6-982b-0394e48bf9c2.png)
![tert-Butyl (R)-3-(2-acetamidopropan-2-yl)-6-chloro-5-methyl-2,3-dihydrospiro[indene-1,4′-piperidine]-1′-carboxylate](/deepweb/assets/sigmaaldrich/product/structures/719/283/e2c466e0-b4df-4961-9844-bfffbe187f3e/640/e2c466e0-b4df-4961-9844-bfffbe187f3e.png)