Alle Fotos(1)
Wichtige Dokumente
553751
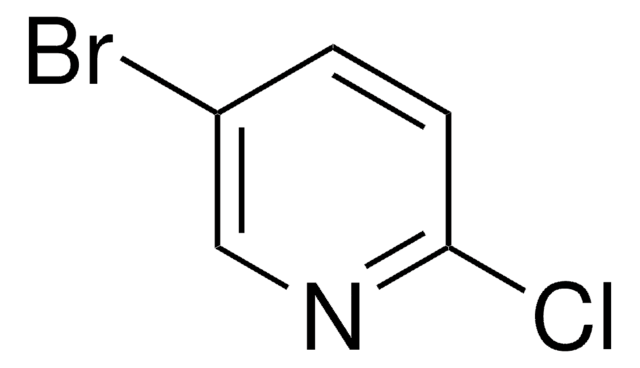
3-Brom-2-chlorpyridin
98%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C5H3BrClN
CAS-Nummer:
Molekulargewicht:
192.44
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
98%
Form
solid
mp (Schmelzpunkt)
54-57 °C (lit.)
Funktionelle Gruppe
bromo
chloro
SMILES String
Clc1ncccc1Br
InChI
1S/C5H3BrClN/c6-4-2-1-3-8-5(4)7/h1-3H
InChIKey
HDYNIWBNWMFBDO-UHFFFAOYSA-N
Allgemeine Beschreibung
3-Bromo-2-chloropyridine can be synthesized from 3-amino-2-chloropyridine or 2-chloro-3-pyridinamine.
Anwendung
3-Bromo-2-chloropyridine may be used to synthesize:
- acetylenic dipyridone
- 3-ethynyl-2-(phenylmethoxy)-pyridine
- nemertelline
- ortho-chlorodiheteroarylamine4 or 2-chloro-N-(2,3,7-trimethylbenzo[b]thien-6-yl)pyridin-3-amine
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Use of hydrogen bonds to control molecular aggregation. behavior of dipyridones and pyridone-pyrimidones designed to form cyclic triplexes.
Boucher E, et al.
The Journal of Organic Chemistry, 60(5), 1408-1412 (1995)
Maria-João R P Queiroz et al.
Bioorganic & medicinal chemistry, 14(20), 6827-6831 (2006-07-18)
ortho-Chlorodiarylamines in the 2,3,7-trimethylbenzo[b]thiophene series were prepared in high yields (70-85%) by C-N palladium-catalyzed cross-coupling using P(t-Bu)(3) as ligand and NaOt-Bu as base. A palladium-assisted C-C intramolecular cyclization of the coupling products gave thienocarbazoles and the dechlorinated diarylamines. Studies of
Synthesis of the first thieno-d-carboline: Fluorescence studies in solution and in lipid vesicles.
Queiroz MJRP, et al.
Journal of Photochemistry and Photobiology A: Chemistry, 181(2), 290-296 (2006)
Alexandre Bouillon et al.
The Journal of organic chemistry, 68(26), 10178-10180 (2003-12-20)
Regioselective and univocal Suzuki cross-coupling reactions performed on halopyridinyl boronic acids provide a flexible and versatile route to a multigram scale synthesis of 2,2'-dichloro-3,4'-bipyridine 14, which allows couplings with excess pyridin-3-yl boronic acid to give a new and efficient two-step
Synthesis of novel halopyridinylboronic acids and esters. Part 2: 2, 4, or 5-Halopyridin-3-yl-boronic acids and esters.
Bouillon A, et al.
Tetrahedron, 58(17), 3323-3328 (2002)
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.



![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)