Alle Fotos(2)
Wichtige Dokumente
472654
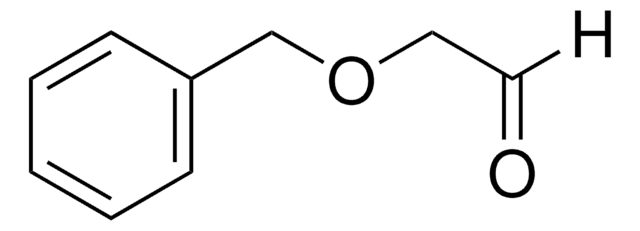
N-Boc-2-aminoacetaldehyd
95%
Synonym(e):
N-(2-Oxoethyl)-carbamidsäure-tert-butylester
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Lineare Formel:
HCOCH2NHCO2C(CH3)3
CAS-Nummer:
Molekulargewicht:
159.18
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
95%
Brechungsindex
n20/D 1.455 (lit.)
Funktionelle Gruppe
aldehyde
amine
Lagertemp.
−20°C
SMILES String
CC(C)(C)OC(=O)NCC=O
InChI
1S/C7H13NO3/c1-7(2,3)11-6(10)8-4-5-9/h5H,4H2,1-3H3,(H,8,10)
InChIKey
ACNRTYKOPZDRCO-UHFFFAOYSA-N
Angaben zum Gen
human ... CTSK(1513)
Allgemeine Beschreibung
N-Boc-2-aminoacetaldehyde is an organic building block. It reacts with Horner-Wadsworth-Emmons (HWE) reagent to afford γ-aminobutyric acid (GABA)-derived α-keto amide/ester units.
Anwendung
α-Methylenation of this amino aldehyde proceeds in a quick and efficient manner using a recently reported protocol involving formaldehyde and catalysis by either pyrrolidine proprionic acid or the dipeptide L-Pro-β-Ala.
Also used in a three-component synthesis of pyrrolidines involving 1,3-dipolar cycloaddition.
Also used in a three-component synthesis of pyrrolidines involving 1,3-dipolar cycloaddition.
A building block in the synthesis of a protected pyrroloproline.
N-Boc-2-aminoacetaldehyde may be employed in the following:
- As a starting reagent in the total synthesis of (+)-negamycin.
- Synthesis of (E)-ethyl 4-((tert-butoxycarbonyl)amino)but-2-enoate.
- Synthesis of 2,2′-bipyridine.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
235.4 °F - closed cup
Flammpunkt (°C)
113 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Efficient total synthesis of (+)-negamycin, a potential chemotherapeutic agent for genetic diseases.
Yoshio Hayashi et al.
Chemical communications (Cambridge, England), (20)(20), 2379-2381 (2008-05-14)
Herein, we describe an efficient strategy for the total synthesis of (+)-negamycin using commercially available achiral N-Boc-2-aminoacetaldehyde as starting material with 42% overall yield for a limited number of steps.
Diethyl [3-Cyano-2-Oxo-3-(Triphenylphosphoranylidene) propyl] phosphonate: A Useful Horner-Wadsworth-Emmons Reagent for alpha-Keto (Cyanomethylene)-triphenylphosphoranes from Carbonyl Compounds.
Lee K.
Bull. Korean Chem. Soc., 28(10), 1641-1641 (2007)
Anniina Erkkilä et al.
The Journal of organic chemistry, 71(6), 2538-2541 (2006-03-11)
A rapid and extremely convenient method for alpha-methylenation of aldehydes with aqueous formaldehyde is described. Two optimal catalytic systems are presented that allow short reaction times and afford the functionalized products in good to excellent yields (up to 99%) and
Anna Turetsky et al.
Scientific reports, 4, 4782-4782 (2014-04-25)
A number of Bruton's tyrosine kinase (BTK) inhibitors are currently in development, yet it has been difficult to visualize BTK expression and pharmacological inhibition in vivo in real time. We synthesized a fluorescent, irreversible BTK binder based on the drug
The Journal of Organic Chemistry, 70, 10869-10869 (2005)
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.