Alle Fotos(1)
Wichtige Dokumente
148830
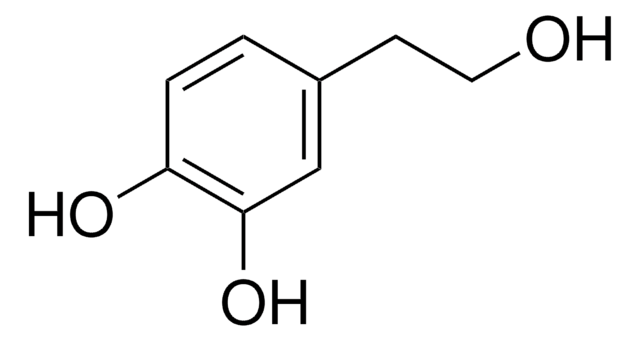
Homovanillylalkohol
99%
Synonym(e):
4-Hydroxy-3-methoxyphenyl-ethanol
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Lineare Formel:
HOC6H3(OCH3)CH2CH2OH
CAS-Nummer:
Molekulargewicht:
168.19
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
99%
Form
solid
mp (Schmelzpunkt)
40-42 °C (lit.)
Funktionelle Gruppe
hydroxyl
SMILES String
COc1cc(CCO)ccc1O
InChI
1S/C9H12O3/c1-12-9-6-7(4-5-10)2-3-8(9)11/h2-3,6,10-11H,4-5H2,1H3
InChIKey
XHUBSJRBOQIZNI-UHFFFAOYSA-N
Verwandte Kategorien
Allgemeine Beschreibung
Homovanillyl alcohol is a key component of Queen mandibular pheromone.
Anwendung
Homovanillyl alcohol was used in the preparation of galactosides.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
235.4 °F - closed cup
Flammpunkt (°C)
113 °C - closed cup
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kyle T Beggs et al.
Current biology : CB, 19(14), 1206-1209 (2009-06-16)
Queen mandibular pheromone (QMP) is produced by honey bee queens and used to regulate the behavior and physiology of their nestmates. QMP has recently been shown to block aversive learning in young worker bees, an effect that can be mimicked
D J Edwards et al.
Biochemical pharmacology, 34(8), 1255-1263 (1985-04-15)
The effects in rats of intraventricular injections of 6-hydroxydopamine (6-OHDA) on the urinary excretion 1-3 weeks later of 3-methoxy-4-hydroxyphenethylene glycol (MHPG), 3,4-dihydroxyphenethanol (DHPE), 3-methoxy-4-hydroxyphenethanol (MHPE), p-hydroxyphenylglycol (pHPG), homovanillic acid (HVA) and 3,4-dihydroxyphenylacetic acid (DOPAC) were examined. The excretion of MHPG
Effects of amphetamine and amfonelic acid on the disposition of striatal newly synthesized dopamine.
H H Miller et al.
European journal of pharmacology, 78(1), 33-44 (1982-02-19)
A comparison of the in vivo biochemical actions of the psychotomimetic central stimulants, d-amphetamine (d-AMPH) and amfonelic acid (AFA), on the metabolism of rat striatal newly synthesized [3H]dopamine (DA) was made by pulse labeling with [3H]tyrosine. No evidence for the
Guangmiao Fu et al.
Current medicinal chemistry, 15(25), 2592-2613 (2008-10-16)
Natural products have long been regarded as excellent sources for drug discovery given their structure diversity and wide variety of biological activities. Phenylethanoid glycosides are naturally occurring compounds of plant origin and are structurally characterized with a hydroxyphenylethyl moiety to
M Phillippe et al.
American journal of obstetrics and gynecology, 143(7), 782-787 (1982-08-01)
Previous investigation has demonstrated biologically significant concentrations of catecholamines in amniotic fluid, which increase with gestation. The half life, metabolic clearance rate, and metabolic fate of these hormones in the amniotic compartment are yet to be established. This study was
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.