17988
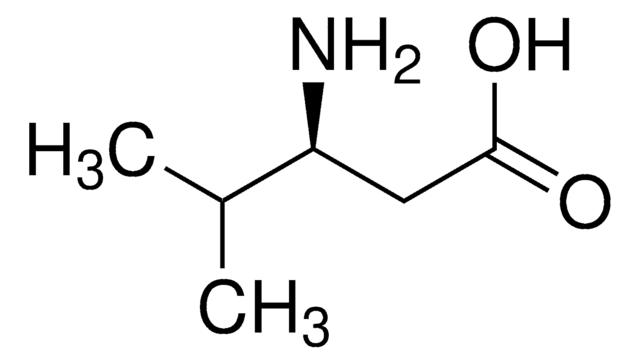
DL-β-Leucine
≥98.0% (NT)
Synonym(s):
(±)-3-Amino-4-methylpentanoic acid, (±)-3-Amino-4-methylvaleric acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H13NO2
CAS Number:
Molecular Weight:
131.17
Beilstein:
1747371
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
Recommended Products
Assay
≥98.0% (NT)
application(s)
peptide synthesis
SMILES string
CC(C)C(N)CC(O)=O
InChI
1S/C6H13NO2/c1-4(2)5(7)3-6(8)9/h4-5H,3,7H2,1-2H3,(H,8,9)
InChI key
GLUJNGJDHCTUJY-UHFFFAOYSA-N
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Beta-leucine and the beta-keto pathway of leucine metabolism.
J M Poston
Advances in enzymology and related areas of molecular biology, 58, 173-189 (1986-01-01)
N E Ward et al.
The Journal of nutrition, 118(2), 159-164 (1988-02-01)
This study was designed to investigate a postulated relationship between vitamin B-12 and leucine metabolism in mature domestic chickens. Plasma amino acid analysis revealed the presence of beta-leucine at a concentration of 60 to 80 mumol/l. After 425 d on
Beta-amino acids: mammalian metabolism and utility as alpha-amino acid analogues.
O W Griffith
Annual review of biochemistry, 55, 855-878 (1986-01-01)
P E Coffey et al.
Chemical communications (Cambridge, England), (22)(22), 2330-2331 (2002-09-21)
Poly-beta-leucines have been evaluated as catalysts for the Juliá-Colonna asymmetric epoxidation of enones; the beta 3-isomer was found to be an effective catalyst for the epoxidation of chalcone (70% ee) and some analogues.
Cobalamin-dependent leucine and beta-leucine synthesis in higher animals.
Nutrition reviews, 39(6), 244-246 (1981-06-01)
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service