902489
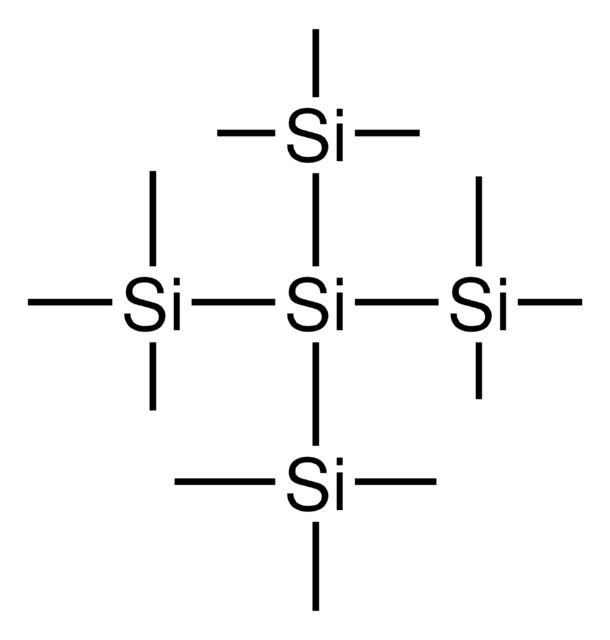
Tris(trimethylsilyl)silanol
≥95%
Synonym(s):
(Hydroxy-bis(trimethylsilyl)silyl)-trimethylsilane, (TMS)3SiOH, 1,1,1,3,3,3-Hexamethyl-2-(trimethylsilyl)trisilan-2-ol, Supersilanol
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C9H28OSi4
CAS Number:
Molecular Weight:
264.66
MDL number:
UNSPSC Code:
12161700
NACRES:
NA.22
Recommended Products
Assay
≥95%
form
liquid
reaction suitability
reaction type: C-C Bond Formation
refractive index
n/D 1.496
density
0.859 g/mL
InChI
1S/C9H28OSi4/c1-11(2,3)14(10,12(4,5)6)13(7,8)9/h10H,1-9H3
InChI key
ABTWCNHNRLMBFR-UHFFFAOYSA-N
Application
Under a dual catalytic copper/photoredox manifold, this supersilanol has been demonstrated by the MacMillan lab to be an excellent reagent for the trifluoromethylation of alkyl halides and aryl halides to yield alkyl-CF3 and aryl-CF3 in high yields. In both cases, these reactions exhibit wide substrate scope with good functional group tolerance. More specifically, a variety of 5-membered and 6-membered heteroaryl halides can be readily converted to the corresponding trifluoromethylheteroarenes under mild conditions. To be use in conjunction with dMesSCF3 (901466) and Ir photocatalyst (902217 or 902225).
related product
Product No.
Description
Pricing
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
200.3 °F
Flash Point(C)
93.5 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Timothy J Boyle et al.
Inorganic chemistry, 57(15), 8806-8820 (2018-07-07)
In an effort to generate single-source precursors for the production of metal-siloxide (MSiO x) materials, the tris(trimethylsilyl)silanol (H-SST or H-OSi(SiMe3)3 (1) ligand was reacted with a series of group 4 and 5 metal alkoxides. The group 4 products were crystallographically
Annie J Jiang et al.
Journal of the American Chemical Society, 131(46), 16630-16631 (2009-11-19)
Mo and W MonoAryloxide-Pyrrolide (MAP) olefin metathesis catalysts can couple terminal olefins to give as high as >98% Z-products in moderate to high yields with as little as 0.2% catalyst. Results are reported for 1-hexene, 1-octene, allylbenzene, allyltrimethylsilane, methyl-10-undecenoate, methyl-9-decenoate
Chip Le et al.
Science (New York, N.Y.), 360(6392), 1010-1014 (2018-06-02)
Transition metal-catalyzed arene functionalization has been widely used for molecular synthesis over the past century. In this arena, copper catalysis has long been considered a privileged platform due to the propensity of high-valent copper to undergo reductive elimination with a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![[4,4′-Bis(1,1-dimethylethyl)-2,2′-bipyridine] nickel (II) dichloride](/deepweb/assets/sigmaaldrich/product/structures/471/091/6faa29b1-bf8a-4d87-90b2-4cc55e082620/640/6faa29b1-bf8a-4d87-90b2-4cc55e082620.png)


![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)

![[(TMEDA)Ni(o-tolyl)Cl] 95%](/deepweb/assets/sigmaaldrich/product/structures/236/439/768c916e-994f-47e3-a980-3ca0471317d7/640/768c916e-994f-47e3-a980-3ca0471317d7.png)