All Photos(1)
About This Item
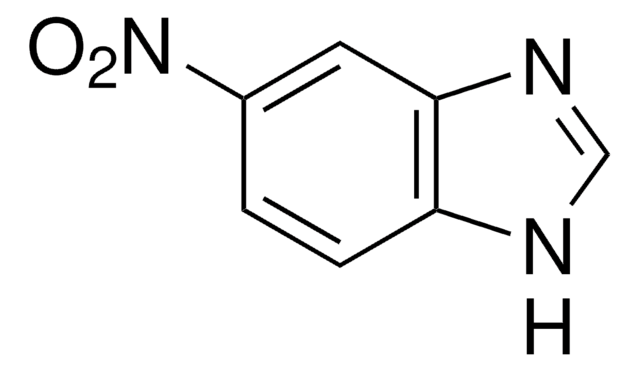
Empirical Formula (Hill Notation):
C3H3N3O2
CAS Number:
Molecular Weight:
113.07
Beilstein:
2815
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
powder
mp
303 °C (dec.) (lit.)
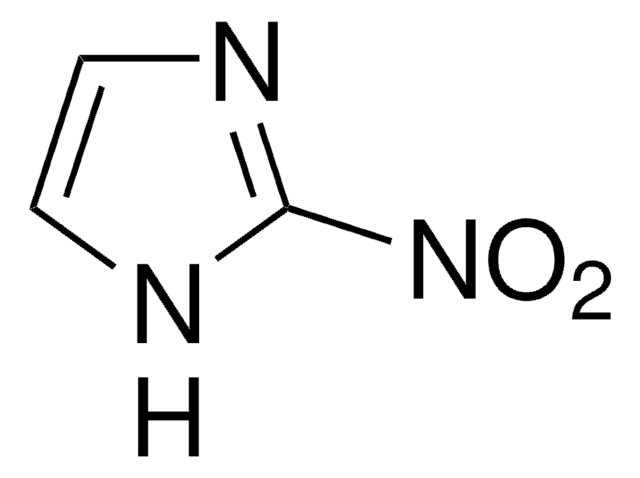
SMILES string
[O-][N+](=O)c1c[nH]cn1
InChI
1S/C3H3N3O2/c7-6(8)3-1-4-2-5-3/h1-2H,(H,4,5)
InChI key
VYDWQPKRHOGLPA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Nitroimidazole is an intermediate during the synthesis of 1-methyl-2,4,5-trinitro imidazole.
Application
4-Nitroimidazole was used in a study to investigate the catalytic efficiency of heterocyclic compounds in the peroxyoxalate chemiluminescence reaction using bis(2,4,6-trichlorophenyl)oxalate as reagent.
related product
Product No.
Description
Pricing
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
392.0 °F - closed cup
Flash Point(C)
200 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Heterocyclic compounds as catalysts in the peroxyoxalate chemiluminescence reaction of bis (2, 4, 6-trichlorophenyl) oxalate.
Jonsson T and Irgum K.
Analytica Chimica Acta, 361(3), 205-215 (1998)
Quantum-chemical studies on thermodynamic feasibility of 1-methyl-2,4,5-trinitroimidazole processes.
Pandurang M Jadhav et al.
Journal of molecular modeling, 19(8), 3027-3033 (2013-04-12)
1-Methyl-2,4,5-trinitro imidazole (MTNI) is a well-known melt cast explosive possessing good thermal stability and impact insensitivity. MTNI has been synthesized from multi-step nitration followed by methylation of imidazole exhibiting low yield. It is desirable to screen the process thermodynamically for
Rayane Mohamed et al.
Journal of agricultural and food chemistry, 56(10), 3500-3508 (2008-05-07)
A nitroimidazole, molecularly imprinted polymer (MIP) was tested to extract four 5-nitroimidazoles (i.e., dimetridazole (DMZ), ipronidazole (IPZ), metronidazole (MNZ), and ronidazole (RNZ)) and three of their metabolites (i.e., DMZOH, IPZOH, and MNZOH) from egg powder samples. Various MIP templates were
Linda A Dunn et al.
International journal of antimicrobial agents, 36(1), 37-42 (2010-05-12)
The 5-nitroimidazole (NI) compound C17, with a side chain carrying a remote phenyl group in the 2-position of the imidazole ring, is at least 14-fold more active against the gut protozoan parasite Giardialamblia than the 5-NI drug metronidazole (MTR), with
Maxime D Crozet et al.
European journal of medicinal chemistry, 44(2), 653-659 (2008-07-02)
To improve the antiparasitic pharmacophore, 20 5-nitroimidazoles bearing an arylsulfonylmethyl group were prepared from commercial imidazoles. The antiparasitic activity of these molecules was assessed against Trichomonas vaginalis, the in vitro cytotoxicity was evaluated on human monocytes and the mutagenicity was
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service