All Photos(1)
About This Item
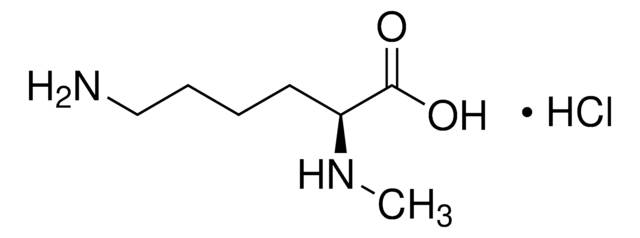
Empirical Formula (Hill Notation):
C7H16N2O2 · HCl
CAS Number:
Molecular Weight:
196.68
EC Number:
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.32
Recommended Products
Quality Level
Assay
≥98.0% (TLC)
optical activity
[α]/D 20.5±1.5°, c = 0.1 in 1 M HCl
storage temp.
2-8°C
SMILES string
Cl.CNCCCC[C@H](N)C(O)=O
InChI
1S/C7H16N2O2.ClH/c1-9-5-3-2-4-6(8)7(10)11;/h6,9H,2-5,8H2,1H3,(H,10,11);1H/t6-;/m0./s1
InChI key
AQELUQTVJOFFBN-RGMNGODLSA-N
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
N ε-methyl-L-lysine was identified as a lysine analog with inhibitory effects on the growth and sporulation of Penicillium chrysogenum and benzyl-penicillin formation by mycelia.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C G Friedrich et al.
Applied and environmental microbiology, 34(6), 706-709 (1977-12-01)
Compounds structurally related to lysine were tested against Penicillium chrysogenum Wis. 54-1255 for inhibition of growth, sporulation, and penicillin formation. This strain is relatively resistant to lysine analogs. The compounds that were the more active inhibitors of growth and whose
Y Minami et al.
Journal of biochemistry, 97(3), 745-753 (1985-03-01)
The amino acid sequence of a ferredoxin from a thermoacidophilic archaebacterium, Sulfolobus acidocaldarius, was determined by a combination of various conventional methods to be as follows: Gly-Ile-Asp-Pro-Tyr-Arg-Thr-His-Lys-Pro-Val-Val-Gly-Asp-Ser-Ser-Gly-His- Lys-Ile -Tyr-Gly-Pro-Val-Glu-Ser-Pro-Lys(Me)-Val-Leu-Gly-Val-His-Gly-Thr-Ile-Val -Gly-Va l-Asp-Phe-Asp-Leu-Cys-Ile-Ala-Asp-Gly-Ser-Cys-Ile-Thr-Ala-Cys-Pro-Val-As n-Val-P he-Gln-Trp-Tyr-Glu-Thr-Pro-Gly-His-Pro-Ala-Ser-Glu-Lys-Lys-Ala-Asp-Pro-V al-Asn- Glu-Gln-Ala-Cys-Ile-Phe-Cys-Met-Ala-Cys-Val-Asn-Val-Cys-Pro-Val-Ala-Ala- Ile-Asp -Val-Lys-Pro-Pro. It was composed
M Friedman et al.
The Journal of nutrition, 111(8), 1362-1369 (1981-08-01)
Growth assays using mice on synthetic amino acid diets showed that substituting epsilon-N-methyl-L-lysine, epsilon-N-dimethyl-L-lysine and epsilon-N-trimethyl-L-lysine for lysine resulted in relative replacement values about 1/12, 1/20 and 1/25, respectively, of that obtained with the standard lysine diet. Similar studies showed
R Garcia et al.
Applied and environmental microbiology, 51(6), 1355-1357 (1986-06-01)
Dansyl derivatives of epsilon-N-mono-, epsilon-N-di-, and epsilon-N-trimethyllysine were resolved from other amino acids in proteins by the use of high-performance liquid chromatography. The system was tested with amino acid standard combinations as well as with acid-hydrolyzed proteins known to contain
M Kushiro et al.
Nephron, 79(4), 458-468 (1998-08-05)
Increases in extracellular matrix (ECM) and changes in its components have been documented in the glomeruli of diabetic nephropathy. Advanced glycation end products formed by glycoxidation have been shown to induce the synthesis of ECM components and transforming growth factor
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service