91095
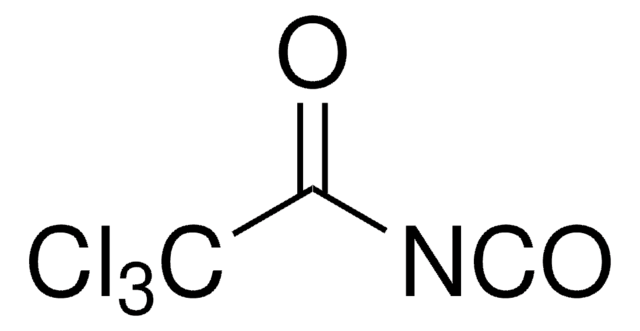
Trichloroacetyl isocyanate
purum, ≥97.0% (GC)
Synonym(s):
2,2,2-Trichloroacetyl isocyanate, alpha,alpha,alpha-Trichloroacetyl isocyanate
About This Item
Recommended Products
grade
purum
Quality Level
Assay
≥97.0% (GC)
form
liquid
refractive index
n20/D 1.480 (lit.)
n20/D 1.480
bp
80-85 °C/20 mmHg (lit.)
density
1.581 g/mL at 25 °C (lit.)
functional group
amine
chloro
isocyanate
storage temp.
2-8°C
SMILES string
ClC(Cl)(Cl)C(=O)N=C=O
InChI
1S/C3Cl3NO2/c4-3(5,6)2(9)7-1-8
InChI key
GRNOZCCBOFGDCL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- One-pot synthesis of 2-acylaminobenzimidazoles: A study on the synthesis of 2-acylaminobenzimidazoles via a reaction between trichloroacetyl isocyanate and 1,2-phenylenediamine derivatives (Shajari et al., 2018).
- Cabamothioate compounds: Research on the synthesis of S-aryl (trichloroacetyl) carbamothioate from a reaction of 2-naphthalenethiol or thiophenol derivatives and trichloroacetyl isocyanate (Shajari et al., 2021).
- Chiral calyx[4]arenes: Diastereoselective synthesis of inherently chiral calyx[4]arenes via reaction of trichloroacetyl isocyanate with 1,3-dihydroxy calixarene (Boyko et al., 2016).
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Eye Dam. 1 - Resp. Sens. 1 - Skin Corr. 1B - Skin Sens. 1
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
150.8 °F - closed cup
Flash Point(C)
66 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service