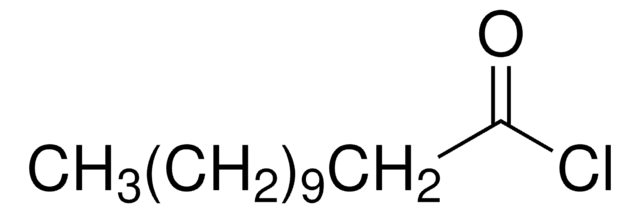61670
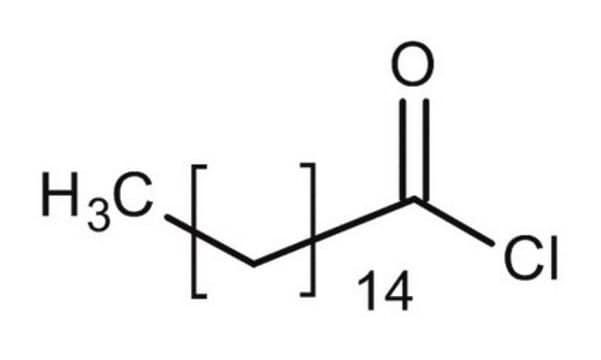
Dodecanoyl chloride
purum, ≥97.5% (GC)
Synonym(s):
Lauroyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)10COCl
CAS Number:
Molecular Weight:
218.76
Beilstein:
1281201
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
purum
Quality Level
Assay
≥97.5% (GC)
refractive index
n20/D 1.445
mp
-13--10 °C
density
0.922 g/mL at 20 °C
functional group
acyl chloride
SMILES string
CCCCCCCCCCCC(Cl)=O
InChI
1S/C12H23ClO/c1-2-3-4-5-6-7-8-9-10-11-12(13)14/h2-11H2,1H3
InChI key
NQGIJDNPUZEBRU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Dodecanoyl chloride aslo known as lauroyl chloride is an acid chloride that can be used as a reagent for the surface modification of:
It can also be used as a:
- Chitosans, by converting it into acylated chitosans for increasing solubility in organic solvents.
- Microfibrillated cellulose (MFC) for improving dispersibility in biopolyamide nanocomposites.
It can also be used as a:
- Starting material for the synthesis of (R)-3-aminotetradecanoic acid (iturinic acid).
- Reagent for the preparation of (3,6-bis(dodecanamido)-2,7-dibromo-9-dodecyl-9H-carbazole).This amide intermediate can be further used in the synthesis of azomethine-bridged ladder-type poly( p-phenylene)s.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
284.0 °F - closed cup
Flash Point(C)
140 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of fully soluble azomethine-bridged ladder-type poly (p-phenylenes) by Bischler- Napieralski reaction
Chen Y, et al.
Macromolecules, 43(24), 10216-10220 (2010)
Novel stereoselective synthesis of (R)-3-aminotetradecanoic acid (Iturinic acid)
Temperini A, et al.
Letters in Organic Chemistry, 6(1), 22-24 (2009)
Characterization of chemical and solid state structures of acylated chitosans
Zong Z, et al.
Polymer, 41(3), 899-906 (2000)
The effect of surface modification of microfibrillated cellulose (MFC) by acid chlorides on the structural and thermomechanical properties of biopolyamide 4.10 nanocomposites
Leszczynska, A, et al.
Industrial Crops and Products, 116(3), 97-108 (2018)
Sanna Virtanen et al.
Carbohydrate polymers, 177, 105-115 (2017-10-01)
Using softwood pulp as the starting material, the synthesis of regioselectively substituted mixed cellulose esters with varying degree of substitution and ratio of short/long chains was successfully completed. The structures of the cellulose esters were characterised. The impact of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service