P78
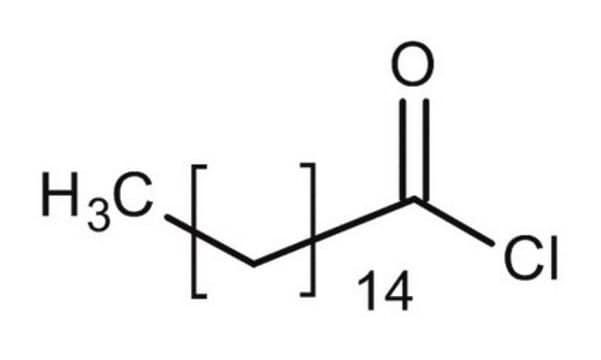
Palmitoyl chloride
98%
Synonym(s):
Hexadecanoyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)14COCl
CAS Number:
Molecular Weight:
274.87
Beilstein:
972409
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.452 (lit.)
bp
88-90 °C/0.2 mmHg (lit.)
mp
11-13 °C (lit.)
density
0.906 g/mL at 25 °C (lit.)
SMILES string
CCCCCCCCCCCCCCCC(Cl)=O
InChI
1S/C16H31ClO/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16(17)18/h2-15H2,1H3
InChI key
ARBOVOVUTSQWSS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Palmitoyl chloride can be used:
It can also be used in the total synthesis of:
- To introduce carbon chain in glycosphingolipid galactosyl ceramide through stereoselective olefin cross-metathesis.
- To prepare monoacyl and 1,3-symmetrical triacylglycerols via regioselective ring opening of an oxirane.
It can also be used in the total synthesis of:
- Hericenone J and 5′ -deoxohericenone C (hericene A).
- Seminolipid.
- Mycobactin S and T equivalents having catechol-glycine group instead of phenol-oxazoline of the naturally occurring mycobactins.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Supplementary Hazards
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
320.0 °F - closed cup
Flash Point(C)
160 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kyoung-Hwa Choi et al.
Carbohydrate polymers, 246, 116487-116487 (2020-08-05)
The purpose of this study was to investigate the improvement in the hydrophobicity of cellulose through gas grafting treatment with long chain fatty acid chloride using high pressure during pressing at high temperature. To do this, the gas grafting treatment
Synthesis and studies of catechol-containing mycobactin S and T analogs
Walz AJ, et al.
Organic & Biomolecular Chemistry, 5(10), 1621-1628 (2007)
Synthesis of the glycosphingolipid β-galactosyl ceramide and analogues via olefin cross metathesis
Rai AN and Basu A
The Journal of Organic Chemistry, 70(20), 8228-8230 (2005)
Total syntheses of seminolipid and its analogues by using 2, 6-bis (trifluoromethyl) phenylboronic acid as protective reagent
Shimada N, et al.
Organic & Biomolecular Chemistry, 17(31), 7325-7329 (2019)
Nrupa Borkar et al.
The Journal of pharmacy and pharmacology, 69(9), 1110-1115 (2017-06-18)
Apomorphine is used to symptomatically treat Parkinson's disease (PD). Oral delivery of apomorphine is generally limited by its short plasma half-life and a hepatic first-pass metabolism. This study was aimed at evaluating the behavioural response of apomorphine and its prodrug
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service