I14150
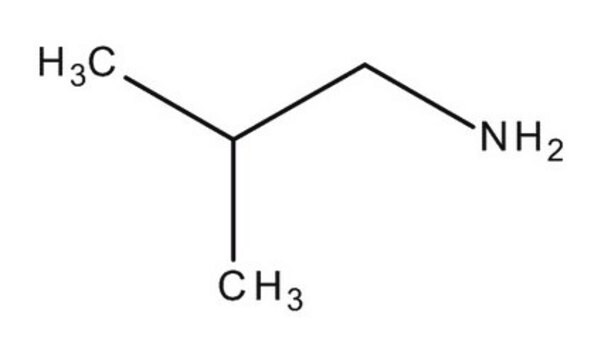
Isobutylamine
99%
Synonym(s):
1-Amino-2-methylpropane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2CHCH2NH2
CAS Number:
Molecular Weight:
73.14
Beilstein:
385626
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.397 (lit.)
bp
64-71 °C (lit.)
mp
−85 °C (lit.)
density
0.736 g/mL at 25 °C (lit.)
SMILES string
CC(C)CN
InChI
1S/C4H11N/c1-4(2)3-5/h4H,3,5H2,1-2H3
InChI key
KDSNLYIMUZNERS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Isobutylamine may be used in the synthesis of N-i-butyl-9(Z),12(Z),15(Z)-octadecatrienamide by reacting with trilinolenin. It can also react with tropolone to form a hydrogen-bonded complex, isobutylammonium 7-oxocyclohepta-1,3,5-trien-1-olate. Fullerene (C60) can undergo oxyamination with isobutylamine to form polyamines.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Flam. Liq. 2 - Skin Corr. 1A
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
15.8 °F - closed cup
Flash Point(C)
-9 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The tropolone?isobutylamine complex: a hydrogen-bonded troponoid without dominant ??? interactions.
Vealey ZN
Acta Crystallographica Section C, Structural Chemistry, 72(10), 730-737 (2016)
A new window on fullerene chemistry: An electrospray mass spectrometric study of the amination of C60.
Wilson SR
Journal of Mass Spectrometry : Jms, 29(4), 186-191 (1994)
Synthesis of a fatty tetrahydroxyamide using peroxygenase from oat seeds.
Piazza GJ
Journal of the American Oil Chemists' Society, 81(10), 933-937 (2004)
Chun-Zhao Liu et al.
Journal of agricultural and food chemistry, 54(22), 8456-8460 (2006-10-26)
Inoculation of leaf explants of Echinacea purpurea (Moench) with Agrobacterium rhizogenes induced hairy roots with the capacity to produce biologically active caffeic acid derivatives (CADs), especially cichoric acid. The kinetics of growth, the uptake of macronutrients, and the accumulation of
L Wang et al.
Journal of immunology (Baltimore, Md. : 1950), 167(11), 6195-6201 (2001-11-21)
Whereas cytokine production in alphabeta T cells is rapidly regulated by exposure to peptide Ag, the mechanisms regulating cytokine production by gammadelta T cells are unknown. In this study, we demonstrate that human Vgamma2Vdelta2 T cells produce IFN-gamma and TNF-alpha
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service